In a recent paper, Ziyang, et al., have produced novel insights into and further developed an HIV vaccine through the use of a unique trimer in order to develop Tier-2 neutralizing antibodies within a murine model.
HIV, a deadly virus that researchers today are still trying to understand, has killed 36.3 million people worldwide. A vaccine, key to preventing the spread and combatting severity of the virus, is still far from development. However, in this present study published in Nature Communications, researchers have made significant strides into the development of a promising vaccine therapy.
In the past it was extremely expensive and time-consuming to investigate the efficacy of these types of antibodies within animal models, but in this study they have established a faster method, developing a new model to produce Tier-2 neutralizing antibodies in mice. Engineering the trimer into DNA for transfection into mice, this method induces antigen production within the host without the need to manufacture a vaccine. Through comparison between mice who received the DNA-encoded native-like trimer with mice who received a standard protein immunisation, they reported that only the mice receiving the DNA-encoded native-like trimer developed Tier-2 neutralizing antibodies (Figure 1).
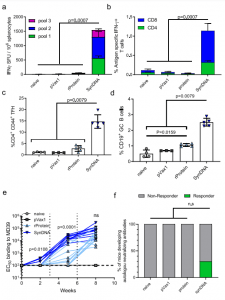
Figure 2: DNA immunization of BG505.MD39 induced stronger T-cell responses and NAb responses than protein immunization in BALB/c mice. All mice in this panel were immunized at Wks 0, 3, 6 with 25 ug DNA or 25 ug RIBI-co-formulated protein and euthanized 2 weeks post the final vaccination for cellular analyses. a, b Comparison of BG505 Env-specific cellular responses in naïve mice, or mice immunized with pVAX plasmid backbone, RIBI-coformulated protein BG505.MD39, or DNA-encoded BG505.MD39 by IFNγ ELIspot assay (a) or intracellular cytokine staining (b). c Frequencies of CXCR5 + PD1 + Tfh cells amongst CD4 + CD44 + cells in the draining lymph nodes in naïve mice or 10 days post pVAX1, protein MD39 or DNA BG505.MD39 immunization. d Frequencies of GL7 + GC B cells amongst CD19 + B cells in the draining lymph nodes in naïve mice or 10 days post pVAX1, protein BG505.MD39 or DNA BG505.MD39 immunization. e Trimer-specific binding antibody responses induced in mice vaccinated with protein or DNAencoded BG505.MD39 post the first, second and third immunization as determined by ELISA. f Frequencies of mice that developed autologous BG505.T332N neutralizing antibodies (ID50 titers greater than 1:45) in naïve mice or mice immunized with pVAX1, protein BG505.MD39 or DNA-encoded BG505.MD39 two weeks post the final vaccination. The dashed vertical line in e refers to the immunization timepoints. Two independent experiments were performed for each panel in the figure. N = 10 mice/group for protein or DNA-encoded BG505.MD39, N = 5 mice/group for naïve mice or pVAX1 treated mice (a, b, e, f); N = 5 mice/group (c, d); each dot or line represents an animal. Error bar represents standard deviation. Center of the error bar represents the mean. Two-tailed Mann–Whitney Rank test used to compare groups; p-values were adjusted for multiple comparison for panels (a–d). Twotailed Mann–Whitney Rank tests were used to compute the p-values comparing EC50 titers between protein and DNA groups for each specific timepoint in e. Two-sided Fisher Exact Tests were used to compare proportion of responders between SynDNA group and naïve/ pVAX1/ protein groups in f. ns not significant. Source data are provided as a Source Data file (Ziyang, et al., 2022).
Following confirmation of the production of Tier-2 antibodies, Ziyang, et al., proceed to investigate the atomic structure of one Tier-2 neutralizing monoclonal antibody using cryo-EM. They were able to describe how the antibody is able to neutralize the virus providing insight into how one may design new vaccines that can generate broadly neutralising antibody responses to the C3V5 epitope.
In such a promising study, engineering and delivering these immunogens via nucleic acid technology into a vaccinated animal, is a massive step in the right direction. These in vivo self-assembly of structurally designed immunogens may allow us the ability to tailor certain vaccines.
Journal article: Ziyang, X, et al., 2022. Induction of tier-2 neutralizing antibodies in mice with a DNA-encoded HIV envelope native like trimer. Nature Communications.
Summary by Stefan Botha










