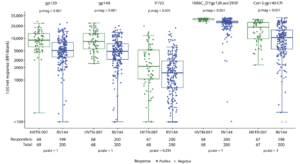
Figure 4C Gray et al., 2019: Geometric mean IgG binding antibody responses among vaccine recipients in the per-protocol cohorts of HVTN 097 and RV144 at the peak immunogenicity time point. Fisher’s exact test P values comparing response rates (p.rate) and Wilcoxon rank sum test P values comparing magnitudes (p.mag) among positive responders between HVTN 097 and RV144 vaccine recipients are provided. Boxplots are based on positive responders only represented by the green and blue circles for HVTN 097 and RV144, respectively; negative responders are shown as gray triangles.
HIV prevalence and incidence is decreasing globally. Improvements in treatment and prevention strategies is not enough to curb the pandemic. The ALVAC-HIV and AIDSVAC B/E vaccine tested in the RV144 trial (Thailand), is the only HIV vaccine to demonstrate partial efficacy. The RV144 vaccine regimen contains HIV immunogens specific for subtype B and E, the predominant subtypes in South-East Asia. Amazing results from the RV144 trials indicated the potential of this vaccine, thus Gray et al., 2019 designed a study (HVTN097) to determine safety and immunogenicity of the RV144 vaccine regimen in South Africans.
Why test an HIV-subtype B/E vaccine in a region where HIV-subtype C is prevalent? Researchers wanted to know if the vaccine regimen could induce similar responses regardless of geographical location. Additionally, HVTN097 was a precursor to HVTN100 which tested an adapted RV144 vaccine regimen that included immunogens from HIV-subtype C. These two trials enable comparison of vaccine-induced immune in different regions (RV144 vs HVTN097), as well as comparison of two different vaccine regimen in the same population (HVTN097 vs HVTN100).
Was HVTN097 as immunogenic as RV144? Gray et al., enrolled 100 HIV-uninfected participants, 91 of whom received all four vaccine doses. Vaccination of the RV144 regimen in South Africa induced poly-functional Env-specific CD4 T cells and HIV-specific antibody (Ab) responses. Vaccine-induced HIV-specific Abs were reactive to not only HIV-subtype-B/E immunogens but also cross-reactive to HIV-subtype C antigens. Interestingly, the vaccine regimen in South African individuals had a higher immunogenicity response rate than Thai individuals. Higher response rates was associated with significantly greater frequency and magnitude of cellular and humoral responses of most of the functional features tested. Results generated from HVTN097 were critical to the modification and adaptation vaccine regimen tested in HVTN100, which also demonstrated promising results for the HIV vaccinology field.
Journal Article: Gray et al., 2019. Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa. Science Translational Medicine











