In an unexpected twist, researchers are exploring how a virus known for causing disease might be repurposed to help fight cancer. Specifically, a herpesvirus that naturally infects squirrel monkeys is showing promise as a tool to enhance the effectiveness of T cell–based immunotherapy.
T cells, key players in the immune system, are capable of targeting and destroying both virus-infected and cancerous cells. Despite their potential, T cells often struggle to function in the hostile environment of a tumour, where various suppressive signals can limit their activity and lifespan. Scientists have been working for years to find ways to overcome these obstacles and better direct T cells to eliminate cancer.
One well-known approach is CAR-T cell therapy, which genetically engineers a patient’s own T cells to recognize and attack specific cancer cells. While powerful, this therapy is not without limitations—particularly in maintaining robust T cell activity within tumours.
Researchers are investigating whether viral proteins can help solve this problem. They focused on herpesvirus saimiri, which infects T cells in squirrel monkeys without causing harm (Figure 1). This virus has evolved proteins that can manipulate T cell signalling, potentially providing a way to enhance T cell survival and function.
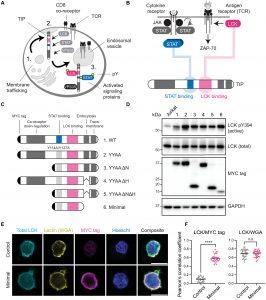
Figure 1: Isolation of an LCK kinase binding and activating peptide. (A) Full-length TIP is trafficked to the plasma membrane (1) where it recruits LCK to co-opt cellular pathways in T cells (2) and is subsequently internalized (3). (B) TIP binds to and activates LCK and recruits STAT proteins to induce their phosphorylation and activation. (C) Truncated TIP variants were generated to isolate a minimal LCK binding region. (D) LCK autophosphorylation (pY394) was assessed in Jurkat T cells by immunoblot to determine the extent of LCK activation. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (E) Microscopy analysis of Jurkat T cells transfected with the minimal TIP construct. Scale bars, 10 μm. (F) Quantification of LCK colocalization with MYC tag or WGA; ****P < 0.0001; not significant (n.s.) P > 0.05; two-tailed t test. All data are representative of three independent experiments (n = 3).
The researchers engineered a version of one such viral protein—tyrosine kinase interacting protein—to bind with LCK, a kinase involved in early T cell activation. This interaction was designed to trigger STAT5, a transcription factor known to support T cell persistence and anti-tumor activity, mimicking the natural effects of cytokines like IL-2 that activate the JAK-STAT5 pathway.
In preclinical mouse models of melanoma and lymphoma, this modified protein successfully sustained T cell activity within tumours, suggesting a novel method to reinforce immune responses against cancer.
Journal article: Yating Zheng, Y. et al., 2025. An engineered viral protein activates STAT5 to prevent T cell suppression. Science Immunology.
Summary by Stefan Botha










