New research has unveiled a fascinating connection between blood leakage into the brain and the activation of harmful genes in microglia, immune cells that reside in the brain (Figure 1). This activation transforms microglia into toxic cells capable of wreaking havoc on neurons, thereby leading to cognitive dysfunction and motor impairments observed in neurological diseases like Alzheimer’s and multiple sclerosis. Even in individuals without dementia, these microglia can contribute to age-related cognitive decline. Consequently, researchers have been diligently seeking to understand the triggers responsible for this transformation, as deciphering the exact mechanisms involved could open doors to novel treatments for various neurological disorders.
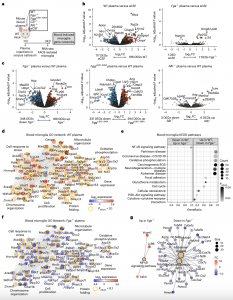
Figure 1: Transcriptional profiling of ligand-selective activation of blood-induced microglial responses in vivo. a, Schematic of experimental design for transcriptional profiling of blood-induced microglial responses. b,c, Volcano plots of DEGs from RNA-seq analysis of sorted microglia from plasma-injected brains. Comparisons between DEGs in microglia of brains injected with WT plasma versus aCSF or Fga −/− plasma versus aCSF (b) and Fga−/− plasma versus WT plasma, Fggγ390–396A versus WT plasma or Alb −/− versus WT plasma (c) are shown. The log2 FC and −log10 adjusted P value cutoffs were log 2 FC > 0.5, adjusted P < 0.1 with Wald test followed by Benjamini–Hochberg (BH) test correction. Top DEGs are shown. Data are from n = 6 Fga−/−, n = 6 WT, n = 6 aCSF and n = 8 Alb−/− mice. d, Coexpression GO networks upregulated in microglia by WT plasma. Adjusted P value <0.1 by hypergeometric test and BH test correction. e, GSEA plots of top upregulated and downregulated pathways in microglia from Fga−/− plasma-injected versus WT plasma-injected brains. Adjusted P value <0.1 by permutation test with BH test correction. f, Overlay of blood microglia GO network with microglial gene expression values from Fga −/− plasma-injected mice. Red shading, genes upregulated in microglia by WT plasma; blue shading, genes downregulated in microglia by Fga −/− plasma; orange border, *P < 0.1 (Wald test followed by BH test correction). g, Coexpression KEGG pathway networks of top upregulated and downregulated pathways in microglia from Fga −/− plasma- injected versus WT plasma-injected brains. Adjusted P < 0.1 by hypergeometric distribution and BH test correction. Padjust, adjusted P value. Created with BioRender.com.
The recent study has pinpointed fibrin, a blood protein known for its role in blood clotting, as the culprit behind the activation of detrimental genes in microglia. Surprisingly, this effect was observed not only in Alzheimer’s disease but also in multiple sclerosis. The discovery suggests that by counteracting the blood toxicity caused by fibrin, it may be possible to safeguard the brain against harmful inflammation and neuronal loss commonly seen in neurological diseases.
Importantly, this study provides a comprehensive understanding of how blood leakage into the brain manipulates the brain’s immune system, resulting in toxic effects during brain diseases. Unraveling the intricate relationship between blood and the brain holds significant potential for the development of innovative treatments targeting neurological conditions.
Additionally, the researchers found that different blood proteins activate distinct molecular processes within microglia. Of all the proteins tested, fibrin stood out as the primary driver of gene and protein activities that render microglia toxic to neurons. The other blood proteins examined did not exhibit the same toxic effects.
The implications of these findings extend beyond specific diseases, as the mechanisms elucidated in this study could be relevant to a broad range of neurological conditions involving blood leaks in the brain. This includes neurodegenerative disorders, autoimmune diseases, stroke, and traumatic brain injury, highlighting the potential for these discoveries to impact diverse areas of neurological research and treatment development.
Journal article: Mendiola, A. S., et al. 2023. Defining blood-induced microglia functions in neurodegeneration through multiomic profiling. Nature Immunology.
Summary by Stefan Botha










