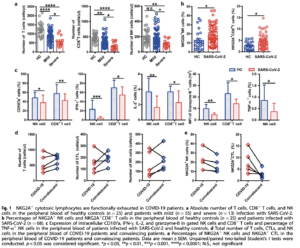
Exhaustion of antiviral T cells in SARS-CoV-2 infection
Natural Killer (NK) cells and CD8+ Cytotoxic T Lymphocytes (CTLs) play key roles in the control of viral infections. Zheng et al have studied the function of these lymphocytes in 68 COVID-19 patients, including 55 and 13 patients with mild and severe disease, respectively. Total numbers of T cells, NK cells and CTLs were reduced in all patients compared to healthy controls, with severe cases having significantly lower proportions than those seen in mild cases. CD8+ T and NK cells from COVID-19 patients had increased expression of the inhibitory receptor NKG2A compared to healthy controls. Furthermore, cells expressing NKG2A had diminished production of CD107a, IFN-γ, IL-2, TNF-α and granzyme B. These findings suggest functional exhaustion of NK and CD8+ T cells and inhibition of antiviral immunity during SARS-CoV-2 infection.
Following antiviral therapy, convalescing patients had an increased number of T cells, CTLs, and NK cells. Importantly, the percentage of NKG2A+ NK and CTLs was reduced, suggesting that downregulation of NKG2A may be crucial for disease control.
A separate study also reported reduced expression of IFN-γ by T helper cells, CTLs and NK cells in severe COVID-19 cases (Chen et al, 2020). Additionally, Qin et al have shown that both helper and cytotoxic T cell subsets are markedly reduced in COVID-19; however, they found no changes in IFN-γ production.
Overall, these data suggest that dysregulation of the immune response, especially exhaustion of T lymphocytes, is a consequence of SARS-CoV-2 infection and may play a role in pathogenesis of the disease. Therapeutic approaches aimed at improving the immune response early on in the infection may, therefore, be beneficial for viral elimination.
References:
- Qin et al., 2020. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clinical and Infectious Diseases.
- Zheng et al., 2020. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cellular & Molecular Immunology
- Zhou et al., 2020. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Annals of Palliative Medicine
Article by Kenneth Omollo