Autophagy, the cellular process of recycling and degrading damaged components or organelles, plays a vital role in various biological systems. In the immune system, it’s particularly involved in processes like B cell self-renewal and the survival of memory and plasma B cells. However, the impact of boosting autophagy in B cells remains somewhat mysterious.
A recent study led by researchers identified a shorter isoform of the protein Rubicon called RUBCN100. This isoform was found to enhance autophagy in B cells (Figure 1). Notably, mice lacking the longer isoform, RUBCN130, generated more memory B cells, a process dependent on autophagy. These findings significantly contribute to our understanding of Rubicon’s role in autophagy.
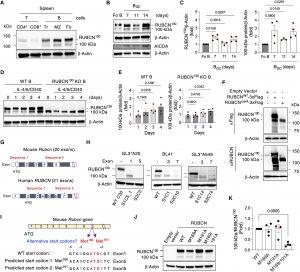
Figure 1: Identification of a RUBCN100 isoform in B cells. (A) Representative immunoblot for RUBCN expression in CD4+ T, CD8+ T, transitional (Tr) B, marginal zone (MZ) B, and follicular (Fo) B cells sorted from two spleens from unimmunized WT mice. (B) Representative immunoblot for RUBCN expression in GC B cells sorted from three to five spleens of WT mice at the indicated time points after NP-KLH-Alum immunization. (C) Densitometric quantification of RUBCN protein levels in GC B (BGC) cells. Fold change values indicate the RUBCN protein level normalized to loading control protein in GC B cells, relative to that in Fo B cells (B). n = 4 independent experiments. (D) Representative immunoblot for RUBCN expression in IL-4/5/CD40–stimulated RUBCN KO and WT B cells in vitro. (E) Densitometric quantification of 100-kDa protein level in (D). Fold change values indicate the RUBCN protein levels normalized to loading control protein in stimulated cells, relative to that in nonstimulated cells. n = 4 independent experiments. (F) Representative immunoblot for 3×Flag-tagged full-length or exon4-deleted (Δex4) RUBCN expression in 293T cells. Two independent experiments showed similar results. (G) The design of CRISPR-Cas9 gene editing for generating RUBCN-deficient mouse and human cell lines. Two targeting sequences for each species were selected. (H) Representative immunoblot for RUBCN expression in CRISPR-Cas9–edited mouse GFP-LC3–expressing (GL3+) A20 B cells, human BL41 B cells, and human A549 epithelial cells. “S” indicates targeted sequences in (I) and “C” indicates selected individual subclones. Two independent experiments showed similar results. (I) The mutation sites in the mouse Rubcn gene. (J) Representative immunoblot for RUBCN in 293T cells transiently transfected with the mutated RUBCN expression vectors. (K) Densitometric quantification of RUBCN protein levels in (J); 100-kDa protein levels are normalized to RUBCN130 in transfected groups and presented as fold change relative to the WT group. n = 4 independent experiments. Data are presented as means ± SEM. Two-tailed paired t tests (C and K). Repeated-measures one-way ANOVA plus Holm-Šídák’s multiple comparisons test (D). Exact P values are shown. P < 0.05 was considered to be significant.
Rubicon is a protein known to inhibit autophagy and has far-reaching effects on various health conditions, including fatty liver, kidney fibrosis, brain α-synuclein accumulation, systemic fat atrophy, osteoporosis, and myocardial ischemia/reperfusion injury. However, a deficiency in RUBCN can also lead to metabolic syndrome and reduced Sertoli cell function in mice.
In the study, the scientists pinpointed RUBCN100, which enhances autophagy in B cells. Mice lacking RUBCN130 or expressing RUBCN100 showed increased autophagy in B cells. This, in turn, led to the generation of more memory B cells and suppressed the differentiation of plasmablasts, a type of antibody-producing cell. Enhanced autophagy shifted the fate of B cell differentiation without affecting the formation or survival of germinal centers, which are crucial for the production of high-affinity antibodies.
The researchers discovered that the interaction between the two RUBCN isoforms, RUBCN130 and RUBCN100, is a key regulator of autophagy. Striking the right balance between these isoforms is crucial for maintaining cellular equilibrium and controlling autophagy and mTORC1 activity. RUBCN deficiency and pharmacological enhancement of autophagy can lead to a significant increase in memory B cell generation.
This revelation of RUBCN100 not only reconciles conflicting findings about the functions of RUBCN but also provides insights into the regulation of B cell memory. These findings have the potential to open new avenues for research and therapeutic interventions in conditions related to immune memory and autophagy.
Journal article: Chao-Yuan, CY., et al, 2023. Opposing roles of RUBCN isoforms in autophagy and memory B cell generation, Science Signaling.
Summary by Stefan Botha










