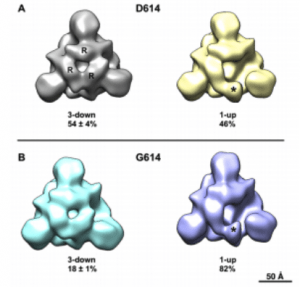
Negative stain electron microscopy reconstructions of expressed Spike constructs following 3D classification. View is looking down the 4-fold trimer axis onto the S1 domain. (A) D614 variant showing the 3-RBD-down structure on the left with individual RBDs labeled (R), and the 1-RBD-up structure on the right with the up RBD labeled (asterisk). Fraction of particle images that sorted into each class indicated below, expressed as average ± standard deviation, N=3 each. (B) G614 variant also showing 3-down and 1-up structures. (Source:
Disclaimer: This is a summary of an article that is in a preprint and has not been peer reviewed.
The D614G mutation in the spike protein of SARS-Co-V2 was described recently and has now become the dominant viral strain in global circulation and has also been shown to result in greater viral infectivity. As various vaccine candidates are now in clinical trials, and which many are based on the D614 original strain, a critical question is whether the G mutated form can result in escape from host neutralizing antibody activity? In a recent pre-peer reviewed paper, authors show that the D614G mutation is in fact more susceptible to host neutralizing antibody activity. This was shown using a combination of mice, nonhuman primates and humans immunized with a nucleoside-modified mRNA-LNP vaccine and one of four different forms of the Spike immunogen. Neutralizing antibody activity was measured in 293T/ACE2 cells using lentivirus particles pseudotyped with full-length SARS-CoV-2 Spike containing either D614 or G614 variant. Vaccinated mice, macaques and humans generated antibody responses that not only recognized the G614 mutation but also had higher titres of neutralization activity to the variant. The possible mechanism giving rise to enhanced neutralization in the variant is the “up formation” of the receptor binding domain in the spike trimer, thereby exposing a greater number of epitopes to host neutralizing antibodies.
D614G Spike Mutation Increases SARS CoV-2 Susceptibility to Neutralization. medRxiv
Also Read:
- Do mutations in the SARS-CoV-2 spike protein enhances viral infectivity?
- Mutation in the Spike protein may explain higher infectivity of SARS-CoV-2 and accelerated disease.
Summary by Clive Gray











