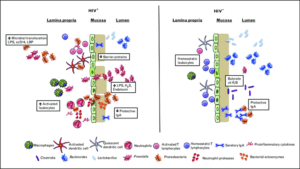
Microbial dysbiosis in HIV is characterized by decreased abundances of Bacteroides, Lactobacillus and beneficial clostridia with increased abundances of Prevotella and pathogenic Proteobacteria, increasing T-cell and DC activation. Increased neutrophil and macrophage accumulation in the lamina propria, bacterial activity in the mucosa, and other mechanisms destabilize the mucosa and GI epithelium, leading to MT and further accumulation of inflammatory microbial products and cytokines in circulation. Source: Lyle-Mckinnon et al., 2015.
Gastrointestinal mucosal damage drives chronic inflammation in HIV infected individuals and restores partially after anti-retroviral treatment (ART). Microbial dysbiosis in the gut of HIV infected patients, could trigger TLR signalling in neutrophils, increase neutrophil survival and contribute to inflammation and mucosal damage. This potential mechanism was investigated by Hensley-McBain et al., 2019.
Neutrophil frequencies were increased significantly in colorectal biopsies from HIV infected-ART suppressed patients compared with HIV uninfected controls. Interestingly, the frequency of non-apoptotic surviving functional neutrophils (CD16hiCaspase-3lo) was increased in biopsies from HIV+ individuals, suggesting that increased neutrophils lifespan could be the cause of increased frequency of neutrophils.
Given that microbes interact with neutrophils and change neutrophils survival, they assessed the association between microbiome alterations ane neutrophils frequency, function and survival. The overall microbiome composition showed a modest association when HIV+ ART-suppressed individuals were compared to HIV- individuals. Sexual orientation was significantly associated with microbiome composition when they compared men who have sex with men (MSM) with non-MSM. Data from previous studies suggested that Prevotella sp. drives neutrophils survival, while Lactobacillus sp. induces apoptosis in myeloid and epithelial cells. Therefore, the relative abundance of Lactobacillus sp. and Prevotella sp. genera as well as the Lactobacillus: Prevotella sp. ratio were compared between HIV+ and HIV- individuals and was significantly lower in HIV infected individuals. Importantly, the Lactobacillus: Prevotella sp. ratio was correlated significantly with neutrophil survival. These findings suggest that the balance of microbiome is perturbed in HIV+ ART-suppressed individuals that may lead to increased neutrophil survival.
Additionally, they incubated blood neutrophils with bacterial species that reported to be altered in HIV infected individuals. While incubation with Lactobacillus sp. reduced survival of blood neutrophils, while Lipopolysaccharides and non-Lactobacillus species increased neutrophil survival. These data provides further evidence that neutrophil survival can be affected based on microbiome composition.
In summary, microbiome alteration in the gut may enhance neutrophil lifespan leading to accumulation of neutrophils in HIV-infected ART-suppressed patients, proposing a mechanism that can be targeted for therapeutic strategies to reduce chronic inflammation in HIV positive individuals.
Journal article: Hensley-McBain et al., 2019. Increased mucosal neutrophil survival is associated with altered microbiota in HIV infection. PLoS Pathog 15(4): e1007672
Article by: Avid Mohammadi










