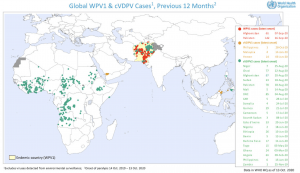
On the 25th of August, Africa was certified polio-free after not reporting a single case of polio in four years. Unfortunately, the world is not yet polio-free as Pakistan and Afghanistan still experience wild type polio epidemics. We currently have polio two vaccines available: oral poliovirus vaccine (OPV also known as Sabin vaccine) and inactivated poliovirus vaccine (IPV). OPV is a live attenuated vaccine that undergoes [limited] replication in the gut inducing robust mucosal immunity. Unfortunately, OPV can regain its replication fitness and sheds via the faecal route, causing Vaccine-associated paralytic polio epidemics and represents a significant risk of polio re-emergence should immunization cease. At present, there is a circulating vaccine-derived poliovirus (cVDPV) in some countries in Africa and South-East Asia (see image).Unfortunately, IPV is less immunogenic than OPV and sole provision of IPV could slow down progress to achieving “polio-free world”.
In response to this global challenge, researchers (Yeh et al., 2020) have modified the Sabin vaccine strain (nOPV2) making it less likely to regain virulence as the original Sabin strain. Specifically, they introduced “modifications in the 5’-UTR that prevent the reversion of a major ‘‘gatekeeper’’ attenuation determinant within domV.” Using cell cultures and animal models they showed that genetic modification of nOPV2 did not affect the thermal stability, replication competency nor the immunogenicity profile the vaccine as compared to the origin Sabin vaccine strain. This vaccine was also tested in a phase 1 trial and demonstrated safety and immunogenicity in IPV-immunised healthy adults (n=15; Van Damme et al, 2019)
Journal Articles:
- Yeh et al., 2020. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host & Microbe
- Van Damme et al., 2019. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. The Lancet.
Also Read: New polio vaccine poised to get emergency WHO approval
Summary by Cheleka Mpande