The coronavirus disease 2019 (COVID-19) pandemic has had devastating effects on global healthcare and economic systems and has led to a high rate of respiratory-related mortality. In addition to pulmonary diseases, SARS-CoV-2, caused by COVID-19, could also bring about multiple organ failure in the form of injury to the liver, heart, kidney, disseminated intravascular coagulation, chronic fatigue, and rhabdomyolysis.
COVID-19 presents itself clinically in varying ways due to localisation of the virus and antigens, degree of inflammation, vascular complications, genetic host factors, age, gender and comorbidities. The immune response of the host also has an impact on disease severity and clinical outcome.
In the majority of COVID-19 cases in children and young adolescents, asymptomatic or mild cases of the disease has been reported. Rare and severe complication of COVID-19 may arise in children between four and six weeks subsequent to the initial infection. This condition is known as a multisystem inflammatory syndrome in children (MIS-C) and presents with high fever, rash, multi-organ dysfunction, and elevated markers of inflammation. The autoantibody profile associated with known autoimmune diseases in patients with COVID-19 or multisystem inflammatory syndrome in children (MIS-C) remains poorly defined. Clinically, MISC-C may manifest itself and be resembling of Kawasaki Disease (KD), a vasculitis that typically affects children. Other symptoms commonly seen in children suffering from MIS-C include gastrointestinal symptoms and myocardial involvement. Treatment of MISC-C and KD includes a high-dose intravenous immunoglobulin (IVIG), corticosteroids, and biologic agents.
It has, in some ways, become a priority of researchers to understand efforts the differences in symptoms and complications seen in COVID-19 patients in order to help combat the pandemic. In recent studies, researchers have identified autoantibodies in the sera of adults and children with COVID-19 and children with MIS-C. The role of high-dose IVIG administration in detecting these autoantibodies has yet to be investigated.
In a recent study, Burbelo, et al., investigated and aimed to characterise the presence of autoantibodies against autoantigens associated with known autoimmune diseases in both adults and children with COVID-19. They collected serum samples from patients infected with COVID-19, healthy control individuals, and children with MIS-C or KD. The sampled serum from patients across multiple countries including the United States, Italy, Chile, and Israel. They made use of the Luciferase Immunoprecipitation Systems assay to detect and quantify the levels of autoantibodies in three different batches of IVIG. It was reported that in terms of age, children with MIS-C had a median age of six years, while children with COVID-19 had a median age of one year. An equal number of males and females were reported among MIS-C patients, while male dominance was found in patients with COVID-19.
Following the assessment of adult patients with COVID-19, they reported high levels of autoantibodies against a number of autoantigens including the lung protein KCNRG, gastric ATPase, and systemic lupus erythematosus (SLE) antigen Sm-D3 (Figure 1). In addition, several other autoantigens namely Ro52, Ro60, La, and GAD65 were detected at lower levels. Increased levels of SmD3, as well as Ro52, Ro60, and La were detected in some adults, however, the levels of both KCNRG and Sm-D3 autoantibodies were found to be high in patients with severe or moderate COVID-19 but not in critically ill patients. Interestingly, in children with COVID-19, low levels or no autoantibodies against KCNRG, Sm-D3, and the other autoantigens that were tested. It was, however, reported in one child that there were high levels of GAD65 autoantibodies, a marker of type 1 diabetes. The patient presented with ketoacidosis and required insulin following admission into hospital.
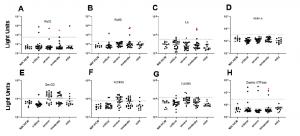
Figure 1: Autoantibodies against known autoantigens in adults with COVID-19 not receiving IVIG. Autoantibody levels against eight well known autoantigens were determined in adults with COVID-19 having varying levels of disease severity: critical, severe, moderate, and mild. Each symbol represents a sample from an individual control subject (SARS-CoV-2 uninfected health care worker (NIH HCW), a patient with COVID-19 (critical, severe, or moderate, or mild). Autoantibody levels are plotted in light units on a log10 scale. The dashed lines represent the cut-off level for determining positive antibody levels for each autoantigen as described in the Methods. Samples from a single patient with moderate disease severity showing antibodies to multiple autoantigens are shown by the red dots (Burbelo, et al., 2021).
In addition to COVID-19 infection being a trigger of certain autoimmune diseases, SARS-CoV-2 infection resulted in both autoantibody-positive and autoantibody-negative diabetes. The study also showed that children suffering from MIS-C lacked La, Ro60, and gastric ATPase autoantibodies (Figure 2). Contrastingly, following IVIG administration, autoantibodies against Ro52, Ro60, La, and gastric ATPase were detected in MIS-C and KD children. The presence of seropositive autoantibodies in IVIG dependent on donor.
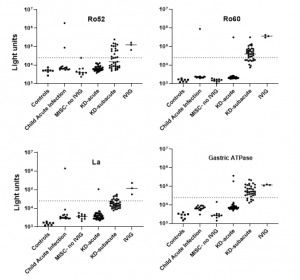
Figure 2: Autoantibodies in the serum of patients with MIS-C and Kawasaki disease (KD) are due to administration of IVIG. Autoantibody levels are plotted in light units on a log10 scale. The dashed lines represent the cut-off level for determining positive antibody titers as described in the Methods. Results from 8 sera from children not infected with SARS-CoV-2 (Controls), 15 sera from children with acute febrile infection not due to COVID-19 or KD (child acute infection), 10 sera from patients with MIS-C who did not receive IVIG (MISC-no IVIG), 39 sera from children with acute KD (KD-acute), 37 sera children with subacute KD (KDsubacute), and three preparations of IVIG are shown (Burbelo, et al., 2021).
Studying the decay of these autoantibodies, in vivo, it was reported that in patients suffering from MIS-C but receiving IVIG, Ro52, Ro60, and La autoantibodies showed a similar decay profile, still present in the blood for several weeks before becoming undetectable by 35-60 days (Figure 3). In contrast, the autoantibodies against gastric ATPase were found to persist for a longer period of time as ATPase is a surface membrane protein that is antibody accessible, which might allow gastric ATPase autoantibodies to bind gastric tissue and thereby slowly be released resulting in persistent presence in the blood.
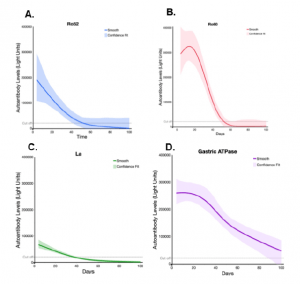
Figure 3: Ro52, Ro60, La, and gastric ATPase autoantibody decay in patients with MIS-C receiving IVIG. Decay plots were generated for Ro52, Ro60, La, and gastric ATPase autoantibody data from the MIS-C (n=21) patients having two or more different time points. Day 0 is the time the patients received IVIG. The solid-colored line for each autoantigen indicates the decay obtained using JMP® 14.0.0 and a smoothing spline (cubic spline) with a λ of 0.77 with the shaded area representing the bootstrap confidence region for each fit. The dotted lines indicate the cut-off value for determining seronegativity for each autoantigen (Burbelo, et al., 2021).
In conclusion in the words of the author:
“In conclusion, unlike adults with COVID-19, our study found no evidence that children with MIS-C or COVID-19 produce autoantibodies associated with known autoimmune diseases. Since we only examined a limited panel of autoantigens, we appreciate that there may be other autoantibodies with MIS-C that we did not detect. In light of recent studies showing increased somatic hypermutation in plasmablasts of MIS-C, additional studies are needed in MISC to determine if specific autoantibodies, play a role in immune complex formation or autoantibody-driven damage to host cells.”
Journal Article: Burbelo, et al. (2021). Autoantibodies Detected in MIS-C Patients due to Administration of Intravenous Immunoglobulin. medRxiv.
Summary by Stefan Botha










