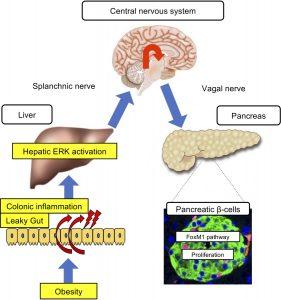
Researchers have discovered that colonic inflammation serves as the earliest trigger in a signalling cascade that drives excess insulin production in obesity (Figure 1). The study, published recently, highlights a novel role of the gastrointestinal tract in regulating glucose balance and opens new avenues for diabetes prevention and therapy.
The pathway from colon to pancreas
Insulin, secreted by pancreatic β-cells, helps cells absorb glucose for energy. In obesity, tissues often become insulin resistant, forcing the pancreas to overproduce insulin. This is partly regulated through a neuronal relay from the hepatic ERK pathway in the liver.
The new findings push the starting point of this cascade even earlier: inflammation in the colon.
- In mouse models, chemically induced colon inflammation activated the hepatic ERK pathway, stimulated neuronal signalling, and increased β-cell numbers, even in non-obese animals.
- In high-calorie diet–induced obese mice, colon inflammation was accompanied by liver ERK pathway activation and β-cell expansion.
- Importantly, reducing colon inflammation blocked liver ERK activation and β-cell proliferation, despite ongoing obesity.
Broader implications
The work identifies colonic inflammation as the missing link that initiates β-cell proliferation during obesity. This finding enhances understanding of how the body maintains glucose homeostasis under metabolic stress and could inform new strategies to:
- Prevent β-cell overstrain in obesity
- Slow the progression to type 2 diabetes
- Develop therapies that target intestinal inflammation as a way to regulate systemic glucose balance
Colonic inflammation acts as the “first domino” in the obesity–diabetes connection, setting off a chain reaction from the gut to the liver to the pancreas that ultimately drives insulin overproduction.
Journal article: Kubo, H., et al. 2025. Colonic inflammation triggers β cell proliferation during obesity development via a liver-to-pancreas interorgan mechanism. JCI Insight.
Summary Stefan Botha