A recent study brings positive findings for patients with severe autoimmune diseases. Researchers tested a new therapy called CD19 CAR T-cell therapy and found promising results in patients with conditions like lupus, myositis, and scleroderma (Figure 1).
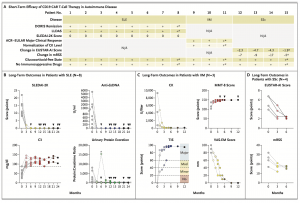
Figure 1: Clinical Efficacy of CD19 CAR T-Cell Therapy in Autoimmune Disease. Panel A summarizes outcomes in 8 patients with sys- temic lupus erythematosus (SLE), 3 patients with idio- pathic inflammatory myositis (IIM), and 4 patients with systemic sclerosis (SSc) treated with CD19 chi- meric antigen receptor (CAR) T cells after 6 months. The asterisks in the columns for Patients 8, 11, and 15 reflect data at 3 months. Scores on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) range from 0 to 105, with higher scores indicating greater disease activity. Scores on the Euro- pean Scleroderma Trials and Research Group activity index (EUSTAR-AI) range from 0 to 10, with higher scores indicating greater disease activity. Values for the modified Rodnan skin score (mRSS) range from 0 to 51, with higher scores indicating worse skin fibrosis. ACR–EULAR denotes American College of Rheumatology– European League against Rheumatism, CK creatine kinase, DORIS Definition of Remission in SLE, LLDAS Lupus Low Disease Activity State, and N/A not applica- ble. Panel B shows long-term outcomes in patients with SLE, including SLEDAI-2K scores and levels of anti–double-stranded DNA (dsDNA) antibodies, com- plement factor 3 (C3), and urinary protein excretion. Arrowheads indicate observation periods of up to 24 months. For the C3 level, the dashed horizontal line in- dicates the lower limit of the normal range. For urinary protein excretion, the dashed horizontal line near the x axis indicates the upper limit of the normal range, and the asterisk indicates a bout of proteinuria that led to kidney biopsy with evidence of lupus nephritis (podocytopathy). The protein:creatinine ratio was cal- culated with urinary protein measured in milligrams and urinary creatinine in grams. Panel C shows long-term outcomes in patients with IIM. Scores on the Manual Muscle Test–8 (MMT-8) range from 0 to 150, with lower scores indicating weaker muscles. Values for the ACR–EULAR Total Improvement Score (TIS) range from 0 to 100, with 0 to 19 indicating no clinical response, 20 to 39 a minimal clinical response, 40 to 59 a moder- ate clinical response, and 60 to 100 a major clinical response. Scores on the visual-analogue scale of extra- muscular symptoms (VAS-EM) range from 0 to 100 mm, with higher scores indicating greater disease activity. Arrowheads indicate observation periods of up to 12 months. Panel D shows long-term outcomes in patients with systemic sclerosis.
Imagine your immune system’s cells equipped with special weapons to target specific enemies. That’s the idea behind CAR T-cell therapy. Scientists take your T cells, modify them with a receptor (CAR) that recognizes specific markers on disease-causing cells, and then reintroduce them into your body. In this case, the CAR targets a marker called CD19 found on certain B cells involved in autoimmune diseases.
Researchers treated 15 patients with severe autoimmune diseases using CD19 CAR T-cell therapy. After preconditioning to prepare their bodies, the patients received a single infusion of the modified T cells.
The study followed the patients for up to two years and found encouraging results:
- All lupus patients achieved remission based on established criteria (Definition of Remission in SLE (DORIS)).
- All myositis patients showed major clinical improvement (American College of Rheumatology-European League against Rheumatism (ACR-EULAR)).
- Scleroderma patients experienced reduced disease activity (European Scleroderma Trials and Research Group (EUSTAR)).
Importantly, these improvements were sustained for a significant period, even after stopping medication.
While it’s too early to say these patients are definitively cured, the study offers exciting potential. CD19 CAR T-cell therapy could offer a long-term, drug-free solution for autoimmune diseases, potentially changing the lives of millions of patients.
Journal article: Müller, F., et al. 2024. CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up, New England Journal of Medicine.
Summary by Stefan Botha










