A treatment originally designed for cancer may hold promise for people living with HIV. A new Phase 1 clinical trial, reports that budigalimab, an immune checkpoint inhibitor targeting programmed cell death protein 1 (PD-1), was safe, well tolerated, and showed early signs of helping the immune system control HIV without daily medication (Figure 1).
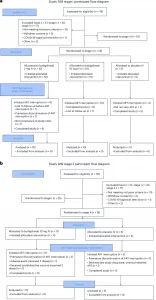
Figure 1: Full CONSORT participant flow diagram for Study M19-939 a) stage I and b) stage II. ART, antiretroviral therapy; IV, intravenous.
The trial marks an important milestone in efforts to achieve a functional cure, a state in which HIV remains controlled in the body without continuous antiretroviral therapy (ART).
HIV remains one of the most persistent viral infections, affecting nearly 40 million people worldwide. While ART suppresses viral replication and prevents disease progression, it cannot eradicate the virus, which hides in long-lived immune cells. Stopping ART usually leads to a rapid viral rebound.
The PD-1 pathway plays a central role in this challenge. In chronic infections like HIV, the PD-1 receptor acts as a biological “brake” on T cells, leaving them exhausted and unable to fight effectively. PD-1 inhibitors, widely used in oncology to unleash immune responses against tumours, offer a way to lift this brake and reinvigorate antiviral immunity.
Budigalimab, developed as a monoclonal antibody against PD-1, was tested in this study at much lower doses than those used for cancer, aiming to safely restore immune function in people living with HIV.
Trial design and key findings
The trial enrolled 41 participants across 11 clinical sites in the United States, Canada, and Australia. Participants received either low-dose budigalimab or placebo and subsequently paused their ART under close medical supervision.
Results were encouraging:
- Six of eleven participants receiving budigalimab in the second stage of the study experienced a delayed viral rebound, while
- Two participants maintained viral suppression for over six months without ART.
No participants in the placebo group experienced this benefit.
Adverse effects were mild to moderate, supporting further development and Phase 2 evaluation. Importantly, the therapy appeared to trigger a more durable immune respons, some participants retained viral control even after budigalimab was no longer detectable in their blood, suggesting that the immune system itself had been strengthened.
The PD-1 pathway was first described by Tasuku Honjo, who received the 2018 Nobel Prize in Physiology or Medicine for this discovery. Since then, PD-1 inhibitors have revolutionized cancer therapy and are now being explored in chronic infections.
Although the effect was limited to a subset of participants, the results point toward a promising new therapeutic strategy. Combining PD-1 blockade with other immunotherapies or latency-reversing agents may further enhance long-term viral control.
This study marks an early but significant step in shifting HIV care from continuous viral suppression to immune-based control, potentially enabling drug-free remission periods in select patients. If confirmed in larger trials, PD-1 immunotherapy could become a cornerstone in the long-term management of HIV, bridging the worlds of oncology, immunology, and infectious disease.
Journal article: Ramgopal M.N., et al. 2025. Budigalimab, an anti-PD-1 inhibitor, for people living with HIV-1: a randomized, placebo-controlled phase 1b study. Nat Med.
Summary by Stefan Botha










