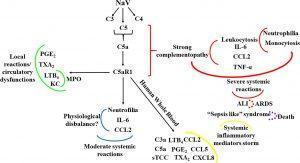
Schematic representation of the contribution of the C5a-C5aR1 axis to local and systemic reactions induced by N. annulifera venom (NaV). NaV acts on complement proteins culminating in anaphylatoxins generation and sTCC assembly. C5aR1 engagement by C5a stimulates LTB4, PGE2 and TXB2 generation, which cause endothelial dysfunctions promoting vascular leakage resulting in extensive edema. In addition, the release of LTB4 and KC, triggered by C5aR1 signaling, may promote the attraction and activation of neutrophils into tissue, increasing MPO levels and potentiating local immunopathology (green lines). The systemic C5aR1 activation, stimulated by NaV sublethal dose injection, promotes moderate increase on CCL2 and IL-6 levels, as well in the neutrophil’s percentage, which could lead envenomated animals to a several physiological disbalance (blue lines). By coupling human whole blood ex vivo (yellow lines) approaches and a severe experimental mouse model of envenomation, it was detected that NaV promotes a hyperacute inflammatory reaction featured by intense systemic inflammatory mediators’ storm, induced by C5a-C5aR1 axis activation. This can evolve to a plethora of pathological conditions including ALI development (red lines) that can progress to ARDS (red dotted lines) and respiratory arrest, a common death cause in humans envenomated by N. annulifera. In addition, these inflammatory mediators can promote organ dysfunction featuring a sepsis like syndrome, which could be a death cause of the envenomated individuals (purple dotted lines). (Source: Silva de França1 et al., 2021)
Envenomation, exposure to a toxin as a result of a bite or sting, can lead to local or systemic immunopathology and in some cases death. Snake bites are responsible for approximately 80000 deaths yearly, and highlights the need for development of novel therapeutics. It is particularly notable that the snakebite envenoming process presents some of the same clinical features observed in certain complement-mediated inflammatory conditions, making this system an interesting therapeutic target.
Researchers from Brazil, Australia and USA explored the complement system contribution to envenomation immunopathology by the snake Naja annulifera (snouted cobra), which occurs in several countries from Sub-saharan Africa and causes accidents in human and dogs.
They observed that N. annulifera venom triggers complement activation mainly mediated by extrinsic routes dependent of the snake venom metalloproteinases action, culminating in anaphylatoxins generation and soluble Terminal Complement Complex assembly. They observed that that C5a anaphylatoxin biding to C5aR1 is responsible for several immunopathological reactions of the envenenomation, which triggers lipid mediators, chemokines and interleukins release in an in vitro human whole blood model.
In an experimental mouse model of envenomation, they demonstrated that C5a anaphylatoxin coupling to C5aR1 caused LTB4, PGE2, TXA2 release in subcutaneous tissue promoting endothelial dysfunctions which caused an extensive oedema. In addition C5a-C5aR1 signalling triggered CXCL1 release and neutrophils infiltration and activation, since high Myeloperoxidase (MPO) levels were detected. C5a-C5aR1 axis activation caused elevation on neutrophils and CCL2 and IL6 systemic levels in mouse exposed to moderate envenomation protocols.
Animals exposed to lethal envenomation protocols presented an intense Acute Lung Injury (ALI) and leukocytosis featured by neutrophilia and monocytosis, which were mediated by the C5-C5aR1 axis, since the treatment these animal with PMX205, a C5aR1 peptidic antagonist, abrogated all these effects.
Researchers concluded that they demonstrated for the first time, that activation of the C5a-C5aR1 axis is the main driver of the local and systemic reactions in envenomation by N. annulifera, a medically important snake on Sub-Saharan Africa.
Journal Article: Silva de França1 et al., 2021. C5a-C5aR1 Axis Activation Drives Envenomation Immunopathology by the Snake Naja annulifera. Frontiers in Immunology.
Summary by Felipe Silva de França










