2020 has been a challenging year, with many breakthroughs in scientific health research. During the year, many putative COVID-19 vaccines were developed of which 57 are currently in human clinical trials. In November, preliminary results in the form of press-releases were made available demonstrating >90% efficacy of COVID-19 vaccines developed by Moderna, Pfizer/BionNTech and Gamaleya. AstraZeneca/Oxford also provided preliminary data, however, the efficacy was much lower <75%. This summary thus aims to provide a brief overview of the BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccines efficacy results published in peer-reviewed journals.
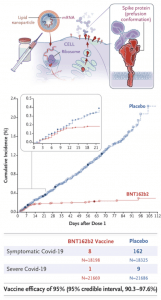
BNT162b2 is a lipid nanoparticle–formulated, nucleoside-modified RNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full-length spike protein co-developed by Pfizer/BioNTech. The mRNA code in this vaccine contains modifications that (1) dampens innate immune sensing resulting in increased mRNA translation and (2) ensures that the spike protein is in the prefusion conformation. Before the phase 3 trial, researchers demonstrated safety and immunogenicity in both young and elderly adults. The phase 3 trial tested the efficacy of two doses, 21 days apart, of either placebo or the BNT162b2 vaccine candidate (30 μg per dose). The recent publication by Polack et al. demonstrated that the BNT162b2 was 95% effective in preventing Covid-19 (95% credible interval, 90.3 to 97.6). They also observed similar vaccine efficacy (generally 90-100%) across subgroups defined by age (57.88% between 16-55 years, sex, race, ethnicity, baseline body-mass index (35.1% obese), and the presence of co-existing conditions. Overall, BTN162b2 was safe and reactogenic, with more BNT162b2 recipients than placebo recipients reported any adverse event (27% and 12%, respectively) or a related adverse event (21% and 5%).
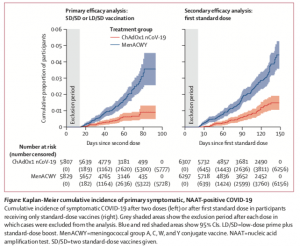
ChAdOx1 nCoV-19 vaccine (AZD1222) consists of the replication-deficient simian adenovirus vector ChAdOx1, containing the full-length structural surface glycoprotein (spike protein) of SARS-CoV-2, with a tissue plasminogen activator leader sequence. This vaccine was also tested in young and elderly individuals induced similar levels of immunogenicity. In the phase 3 trials, majority of individuals were < 55 years (87.8%) with some individuals having comorbidities [(cardiovascular disease (6.7-13.%), respiratory disease (10.4-13%) and diabetes (1.1-3%)].
Participants in the ChAdOx1 nCoV-19 group received two doses (ideally 4 weeks apart) containing 5 × 1010 viral particles (standard dose; SD/SD cohort); a subset in the UK trial received a half dose as their first dose (low dose) and a standard dose as their second dose (LD/SD cohort).
Interestingly, the few vaccinees who received the LD/SD dose had a much higher efficacy (90%) compared to individuals who received the SD/SD dose (62.1%). Unfortunately, determining the immunological mechanism for higher efficacy in the LD/SD is beyond the scope of the published article. However, it does suggest in times of vaccine shortage receiving a lower dose does not negatively impact efficacy. Researchers concluded that results presented in this Article constitute the key findings from the first interim analysis, which are provided for rapid review by the public and policy makers. In future analyses with additional data included as they accrue, we will investigate differences in key subgroups such as older cohorts, ethnicity, dose regimen, and timing of booster vaccines, and we will search for correlates of protection.
Journal Articles:
- BNT162b2: Polack et al., 2020. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. NEJM.
- ChAdOx1 nCoV-19: Voysey et al., 2020. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet
Summary by Cheleka AM Mpande