In a recent paper by Taha, et al., the researchers identified the imidazole-based antifungal, Bifonazole, and characterised its activity against SARS-CoV-2 using high-throughput screening techniques (Figure 1).
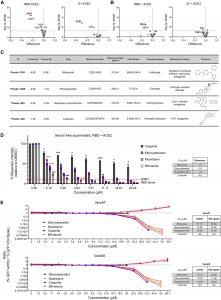
Figure 1: Screen validation identifies bifonazole as the top SARS-CoV-2 blocking candidate. (A and B) Validation (n = 4) of the top 45 candidates using the RBD or the S1 domain of SARS-CoV-2 spike protein following method A (A) or method B (B). (C) Table describing the top 4 candidates selected for subsequent validation, including Z scores obtained following method A or method B. (D) SARS-CoV-2 RBD lysates were incubated as in method B with a dose range (0–25 μM) of ethinylestradiol, nicardipine, cisapride, and bifonazole, followed by addition of ACE2 lysates, and luminescence was measured. Anti-RBD was added as a positive control (dashed line), and the RBD alone (LgBiT, dotted line) was used as a negative control (n = 3–6, two-way ANOVA with Dunnett’s multiple corrections test compared with mock for each drug; orange asterisks signify that all subsequent values have the same level of significance). (E) VeroE6 or Vero-hACE2-TMPRSS2 (VeroAT) cells were treated with non-toxic concentrations of the indicated drugs for 15 min, followed by infection of cells with SARS-CoV-2-spike-pseudotyped VSV (VSV-GFP-Spike) or WT VSV-GFP (MOI 1.0), followed by high-content fluorescence imaging. The ratio of GFP foci obtained for WT VSV-GFP- over VSV-Spike-GFP-infected cells was graphed across the concentrations tested for all four compounds in both cell lines. Tables on the right indicate concentrations that are able to reduce GFP focus counts from WT VSV- or VSV-Spike-infected cells by 50% (n = 5 per condition; see Figures S2G–S2I for raw data) (Taha, et al., 2022).
In this study, researchers made use of small molecules which are used in anti-SARS-CoV-2 therapeutics by characterising and identifying which small molecules were effective at blocking S RBD-ACE2 interactions. The researchers made us of a split-luciferase assay for high-throughput screening of these small molecules.
Following screening of 1,200 compounds, Bifonazole showed the most promise and was a potent competitive inhibitor of SARS-CoV-2 S RBD-ACE2 interactions (READ MORE).
This study highlighted the effectiveness of Bifonazole as an anti-SARS-CoV-2 therapeutic and further highlighted its potential for use following further testing. Bifonazale displayed a high potency and specificity for SARS-CoV-2 inhibition.
Journal article: Taha, Z., et al., 2022. Identification of FDA-approved Bifonazole as SARS-CoV-2 blocking agent following a bioreporter drug screen. Molecular Therapy.
Summary by Stefan Botha










