It has been reported that pregnant women increasingly susceptible to respiratory pathogens, and have an increase likelihood of hospital admission which may lead to ICU admission or fatal complications. This is especially significant in our global battle agains severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In addition, pregnancy-related complications such as pre-term delivery, pre-eclampsia, and placental pathology are being linked to SARS-CoV-2 infection. As the immune system places an essential role in this pathology, the onset of pregnancy induces critical alterations in adaptive and innate immunity whereby the degree and nature of these changes is dependent on gestational age. The effects of SARS-CoV-2 on these alterations has yet to be investigated.
In recent studies, researchers have revealed antibody responses to SARS-CoV-2 specific antigens being detectable 2-3 weeks after the onset of symptoms, and people who have recovered from SARS-CoV2 infection produce and harbour antibodies with a wide range of neutralising activity towards new infection. It is important to consider when repeating vaccination during pregnancy, the prevalence and timing of neonatal immunity after maternal exposure. In the current study, researchers set out to investigate the profile and specificity of maternal serum and neonatal cord blood antibody responses to SARS-CoV-2 virus exposure in mothers.
In a recent study published in a preprint journal, Boelig, et al., collected and processed samples of maternal and cord blood pairs. From the samples, 36 had a known history of COVID-19 during pregnancy (positive PCR test), and of those, 47% were diagnosed with COVID-19 within 30 days after delivery. In addition, positive maternal serology (IgG or IgM to SARS172 CoV-2 spike) was found in 15 of the remaining 76 patients without a confirmed diagnosis. This was reflected in positive cord blood IgG. This equates to a seroprevalence rate of 20% among study participants. 92% percent of the 36 people who had a SARS-CoV-2 infection verified by PCR had positive S-IgM (N=33), and 92% of the 182 (N=33) had positive S-IgG. It was reported that those testing positive for COVID-19 have high SARS-CoV-2 spike protein-specific IgM and IgG levels in their maternal blood and high IgG, but low IgM levels in their cord blood which validates the theory that IgM does not cross the placenta. 20% (15 of 76) of people with no previous COVID-19 diagnosis were seropositive for SARS-CoV-2 spike protein.
In this study they investigated maternal IgG and IgM responses to specific coronavirus spike proteins to see if there was any cross-reactivity between different types of coronaviruses (Figure 1). It was reported that patients who had been exposed to COVID-19 expressed higher IgG responses to SARS-CoV-2 spike, CoV1 spike, and MERS spike in maternal blood and cord blood, indicating a predominantly cross-reactive IgG response. In contrast, IgM responses in maternal blood were more specific to SARS-CoV-2. Interestingly, cord blood expression resembled maternal blood responses for IgG in terms of size and epitope specificity. Lower IgM was detected in cord blood as expected.
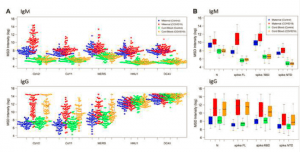
Figure 1: Antibody responses from maternal and cord blood samples collected from 91 pregnant women. Maternal and Cord Blood COVID-19 refers to samples from patients with known COVID-19 history (PCR confirmed) or positive maternal serology. Data reported as natural log-transformed luminescence signal. A) CoV-specific IgM responses (top) and CoV-specific IgG responses (bottom) in maternal sera and cordblood samples to the spike proteins of SARS-CoV1, SARS-CoV2, MERS-CoV, HKU-1, and OC43. Participants with prior COVID-19 history had significantly higher responses not just to CoV2 spike (p < 10-18), but also CoV1 spike (p < 10-9) and MERS spike (p < 10-8). By contrast, for IgM responses in maternal blood, participants with prior COVID-19 history showing significantly higher response to CoV2 (p < 10-19), but to a lesser degree for CoV1 (p < 10-5), and no significant differences for MERS. B) Fine specificity of SARS-CoV-2 specific IgM (top) and IgG ((bottom) responses in maternal sera and cordblood samples to SARS-CoV2 epitopes ,i.e., nucleoprotein (N), the full-length spike protein (spike-FL) and its functional subdomains, i.e., receptor binding domain (RBD) and N-terminal domain (NTD). Subjects with prior COVID-19 infection had robust IgG response in maternal blood to CoV2 N, S (full length) and S (RBD) antigens that were approximately 20-fold, 150-fold, and 10-fold higher, respectively, than what was found in subjects without prior infection, with more modest responses to the S (NTD) antigen. By contrast, maternal blood IgM response to S (full-length) was approximately 20-fold higher in COVID-19 subjects than in subjects with no prior COVID-19 infection history, but only 2- to 3-fold higher for N or S (RBD) (Boelig, et al., 2021).
A PCA plot of IgG and IgM responses to SARS-CoV-2 antigens illustrates that those with exposure to COVID-19 has significant differences from samples without prior exposure and maternal samples can be distinguished from cord blood samples (Figure 2). It was reported that the SARs-CoV-2 spike and N-terminal domain (N) antigens showed good correlation of IgG but not IgM responses between paired maternal and cord blood samples.
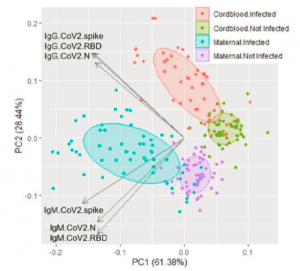
Figure 2: Principal Component Analysis of antibody responses. IgM and IgG responses to CoV2 antigens N, spike, and RBD clearly distinguish between prior COVID-19 cases and maternal from cord blood.
Following maternal exposure to COVID-19, a particular maternal and cord blood antibody profile is expressed. The latency from exposure is related to the serologic profile. It can be concluded that there is sufficient and efficient transfer of IgG antibodies from the mother to the cord blood whereby IgG antibodies cross-react with related CoV-1 and MERS spike epitopes. Contrastingly, IgM antibodies cannot cross the placenta through to the fetus therefore not allowing for the development of neonatal passive immunity. These antibodies are highly specific towards SARS-CoV-2, highlighting qualitative differences between maternal and neonatal passive immunity.
NB to note: medRxiv is a preprint server which publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, or guide clinical practice or treated as established information.
Journal article: Boelig, et al., 2021. Comprehensive Serological Profile and Specificity of Maternal and Neonatal Cord Blood SARS CoV-2 Antibodies. medRxiv.
Summary by Stefan Botha










