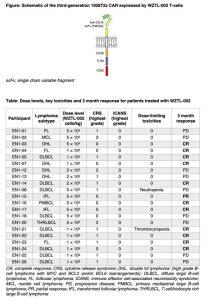
A recent phase 1 dose escalation trial has unveiled compelling data on a new wave of anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, marking a significant leap in the evolution of these therapies (Figure 1).
Current CAR T-cell therapies utilizing anti-CD19 with CD28 co-stimulation, like axicabtagene ciloleucel and brexucabtagene autoleucel, showcase potent efficacy against B-cell non-Hodgkin lymphomas. However, their association with neurotoxicity (immune effector cell-associated neurotoxicity syndrome, ICANS) in nearly half of recipients, along with cytokine release syndrome (CRS) affecting up to 90%, has been a limiting factor.
This groundbreaking study centers on a novel third-generation autologous anti-CD19 CAR T-cell product integrating CD28 with a toll-like receptor 2 (TLR2) co-stimulatory domain. Preclinical investigations reveal that the addition of the TLR2 domain not only maintains or enhances efficacy but also significantly reduces the production of pro-inflammatory cytokines IFN-γ and GM-CSF, which are implicated in CRS and ICANS. Importantly, this reduction in cytokines is compared to CARs with CD28 co-stimulation alone.
These promising trial results represent a significant milestone in advancing novel CAR T technology, propelling the future landscape of CAR T therapies globally. Furthermore, this breakthrough holds promise in addressing unmet needs within markets not yet targeted by major pharmaceutical companies.
The reduced side effect profile exhibited by WZTL-002 CAR T-cell therapy presents a compelling opportunity to address these unmet needs, hinting at a new era of CAR T-cell therapies with potentially improved safety profiles and broader market applications.
Journal article: Weinkove, R., et al. 2023. A Phase 1 Dose Escalation Trial of Third-Generation CD19-Directed CAR T-Cells Incorporating CD28 and Toll-like Receptor 2 (TLR2) Intracellular Domains for Relapsed or Refractory B-Cell Non-Hodgkin Lymphomas (ENABLE). Blood.
Summary by Stefan Botha