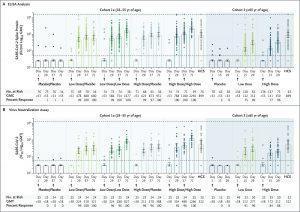
Humoral Immunogenicity: Shown are measures of humoral immunogenicity in serum samples obtained from the participants in cohort 1a (left side) and cohort 3 (right side), according to the receipt of the low or high dose of Ad26.COV2.S or placebo. In cohort 1a, the participants received two injections of high-dose or low-dose vaccine or placebo, as indicated with slashes (e.g., placebo/placebo if they received two injections of placebo). The samples were measured on enzyme-linked immunosorbent assay (ELISA) in ELISA units (EU) per milliliter (Panel A) and on wild-type virus neutralization assay, with seropositivity defined as a half maximal inhibitory concentration (IC50) titer of more than 58 at the lower limit of quantitation (Panel B). Logarithmic values are reported as the geometric mean concentration (GMC) in the ELISA analyses and as the geometric mean titer (GMT) in the neutralizing-antibody analyses. The values were measured at baseline and at day 29 after vaccination in all the participants and on days 57 and 71 in those in cohort 1a. The two horizontal dotted lines in each panel indicate the lower and upper limits of quantitation of the respective assay; values below the lower line have been imputed to half the lower limit of quantitation. 𝙸 bars indicate 95% confidence intervals. HCS denotes human convalescent serum. (Source: Sadoff et al., 2020)
A recent study in the New England Journal of Medicine reports the interim analysis of the phase 1-2a Ad26.COV2.S candidate COVID-19 vaccine is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilised SARS-CoV-2 spike protein. These trials tested a single or two-dose regimen Ad26.COV2.S vaccine at either low (5×1010 viral particles/mL) or high (1×1011 viral particles/mL) doses in younger and older adults.
Overall the vaccine was safe and well-tolerated, with some individuals experiencing local injection site pain and systemic adverse reaction (fatigue, headache, myalgia, fever). Researchers observed higher grade 3 side effects in vaccinees given a high dose. Researchers observed five serious adverse events, one of which was vaccine-related and caused hospitalisation due to fever, while the other four were unrelated (hypotension, bilateral nephrolithiasis, legionella pneumonia, worsening of multiple sclerosis).
The main immunogenicity results presented in the article were vaccine-induced SARS-CoV-2 specific antibody binding affinity to spike protein and neutralisation capacity (Day1, 29, 57 and 71); and CD4 and CD8 functional capacity (Day 1 and 15). Vaccination with both single and two-doses of low or high Ad26.COV2.S vaccine titres induced robust antibodies that exhibited to binding and neutralisation capacity in range with those detected during convalescence. Further, the booster shot given at 56 days did boost vaccine-induced immunity. Analysis of T cell immunity induced detectable CD4 T cells in most adults regardless of age, however vaccine-induced CD8 T cells were less detectable in older compared with younger adults.
Researchers concluded that their “interim analysis indicates that vaccine candidate Ad26.COV2.S is safe and immunogenic in both younger and older adults. This finding, in combination with the results in preclinical challenge studies, has supported our decision to proceed with two phase 3 trials (NCT04505722 and NCT04614948) to evaluate the efficacy of either a single-dose or two-dose regimen of the lower dose (5×1010 viral particles) of Ad26.COV2.S.”
Journal Article: Sadoff et al., 2021. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. NEJM.
Summary by Cheleka AM Mpande










