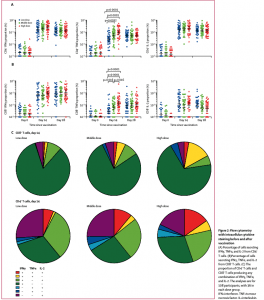
A replication defective Ad5 vectored vaccine expressing the spike glycoprotein of SARS-CoV-2 based on the full-length spike gene from the Wuhan-Hu-1 strain was given to three groups of volunteers at different doses (thirty six volunteers in each group). A single shot was allocated intramuscularly in the arm of the participants in the low dose group (5×10¹⁰ viral particles per 0·5 mL). The participants in the middle dose group received one shot intramuscularly in the arm (1×10¹¹ viral particles per mL). Participants in the high dose group received a double-shot regimen with one vial of the Ad5 vectored COVID-19 vaccine in one arm and two vials of the Ad5 vectored COVID-19 vaccine in the other arm (1·5×10¹¹ viral particles per 1·5 mL). The most common injection site adverse reaction was pain, reported in 58 (54%) vaccine recipients, and the most commonly reported systematic adverse reactions were fever (50 [46%]), fatigue (47 [44%]), headache (42 [39%]), and muscle pain (18 [17%]. Most adverse reactions that were reported in all dose groups were mild or moderate in severity. No serious adverse event was noted within 28 days post-vaccination. The vaccine elicited specific antibody responses to the receptor binding domain as well as neutralizing antibodies to live SARS-CoV-2, with the high does group showing higher antibody titres than the lower dose groups. T cell responses were assessed using the IFNγ ELISPOT assay and appeared to peak at day 14 after vaccination, with responses correlating with vaccine doses. Both CD4+ and CD8+ T cells showed some degree of IFNγ-TNFa-IL-2 polyfunctional responses at day 14 after vaccination, with either IFNγ or TNFa predominating as single or dual-expressing CD8+ and CD4+ T cells, respectively. The authors state in conclusion that “we found that the Ad5 vectored COVID-19 vaccine is tolerable and immunogenic in healthy adults. Specific humoral responses against SARS-CoV-2 peaked at day 28 post-vaccination, and rapid, specific T-cell responses were noted from day 14 after one shot of the vaccine.”
Journal Article: Zhu, Li et al., 2020. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. the Lancet
Summary by Clive Gray