Immunotherapy has emerged as a promising new cancer treatment modality over the last decade. The components of immune-system are the integral part of tumor microenvironment and responsible for the tumor progression and/or regression. Consequently immunotherapeutic approaches involves the modulation of immune cell activity by using adoptive cell transfer (ACT), dendritic cell vaccines, oncolytic viruses, chimeric-antigen receptor T-cells, or monoclonal antibodies (mAbs), etc. Although all these strategies have displayed substantial improvement in clinical outcomes for many types of cancers, the long term benefits remain elusive. Therefore, integrated efforts are necessary to improve the efficacy and attain long term sustainability of anti tumor immune response.
A recent study published in Cell metabolism, Jan 2018, by Mehrotra and colleagues reported an ex vivo culture strategy to improve the long term efficacy of adoptive cell therapy. Using the melanoma murine model, they differentiated CD4+ T cells into hybrid Th1/17 cells that secrete both IL-17 and IFNγ which exhibit superior anti-tumor activity than Th1 and Th17 when adoptively transferred to the tumor bearing mice. In addition, antibody mediated targeting of CD38 (nicotinamide adenine dinucleotide-NAD+- hydrolase) on CD4T cells, rewired the metabolic fitness of tumor infiltrating lymphocytes and improved the tumor control. Chatterjee et al., combined the culture conditions of Th17 (IL6, IL1β, IL23, TGFβlo) and Th1 cells (with IL12 and IL2) to generate hybrid Th1/17 cells, which co-expressed elevated levels of IFNγ and IL17. The intermediate expression of chemokines, effectors molecules, stemness-associated genes and metabolic enzymes by Th1/17 cells may reflect plasticity of Th1 and Th17cells. Nonetheless this unique molecular signature has provided Th1/17 cells an added advantage of increased antitumor activity than Th1 and Th17 cells as shown in mouse ACT models of melanoma and metastatic lung cancer.
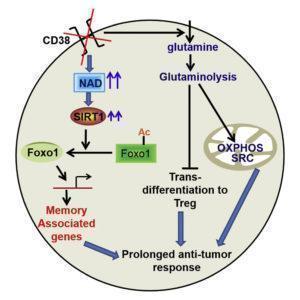
Chatterjee et al., have shown that the hybrid Th1/17 cells exhibit unique metabolic preferences that rely on glutamine-driven oxidative phosphorylation and the shunting of glycolysis into pyruvate to drive the TCA cycle. Inhibition of glutamine metabolism by using glutaminase inhibitor, showed reduced expression of effector molecules and attenuation of anti-tumor response by hybrid Th1/17 cells. Th1 cells primarily use aerobic glycolysis for effector functions, whereas Th17 cells require fatty acid oxidation (FAO). Th1/17 cells have their extracellular acidification rate (ECAR, a measure of aerobic glycolysis) and oxygen consumption rate (OCR) fall within an intermediate range compared to Th1 and Th17cells. These findings suggest that the metabolic preference of hybrid Th1/17 cells to use glutaminolysis provide survival advantage in the tumor environment where availability of glucose is a limiting factor for immune-surveillance.
The levels of NAD+ associate with T cell proliferation, cytokine production and survival. Chatterjee et al., have observed that the increased levels of NAD+ in Th1/17 cells depend upon glutaminolysis which is required for the maintenance of their anti-tumor activity and viability in vivo. Moreover, Th1/17 cells also have increased activity of Sirt1, an epigenetic modulator and NAD+-dependent protein deacetylase. NAD+ dependent Sirt1 deacetylase activity is essential for the anti-tumor activity of hybrid Th1/17 cells, as genetic ablation or pharmacological inhibition of NAD+/ Sirt1 resulted in decrease in the frequency of IL17+IFNγ+ cells, reduced expression of stemness associated molecules and failure to exhibit potent tumor control upon adoptive transfer. In addition, inhibition of Sirt1 activity did not affect the expression of key enzymes associated with different metabolic pathways suggesting that NAD+-Sirt1 axis regulate Th1/17 cell functions, but not their metabolic reprogramming. Further Chatterjee et al have shown that inhibition of NAD+/ Sirt1 increased the acetylation of Foxo1 (transcription factor associated with T cells memory) to attenuate its function thereby affecting Th1/17 cell migration, effector function, stemness, and anti-tumor responses. Phosphorylation of Foxo1 facilitates its nuclear export and loss of transcriptional activity. The authors have observed decreased nuclear retention of Foxo1associated with low antitumor activity compared to Th1/17 cells. Inhibition of AKT in Th1 cells significantly reduced the phosphorylation of Foxo1, and concomitantly increased its nuclear retention. Thus antitumor phenotype of Th1 can be improved by inhibiting the AKT-Foxo1 axis. The data suggested that the increased levels of NAD+-Sirt1-Foxo1 axis in Th1/17 cells is responsible for the enhanced antitumor effector functions compared to Th1 cells.
To improve the anti-tumor response of CD4+ T cells, Chatterjee et al., showed that the levels of NAD+ can be increased by inactivating CD38, a NADase cell surface molecule that is expressed at low levels in Th1/17 cells. Genetic ablation of CD38 or use of blocking anti CD38 mAb in CD4T cells showed phenotype similar to Th1/17 cells including increase in glutamine uptake, glutaminolysis, mitochondrial biogenesis, and improved anti-tumor activity. Interestingly, expression of PD1 (inhibitory receptor associated with T cells exhaustion) was not much different between the tumor infiltrating WT or CD38KO T cells. This implies that CD38 expression plays a key role in metabolically modulating even PD1-expressing exhausted T cells.
The present study on murine model by Chatterjee et al., showed that combining anti-CD38 antibody along with adoptive transferred T cells enhanced anti-tumor response. Th1/17 hybrid cells have emerged as potent candidate for cell therapies for their better and sustainable metabolic adaptation in the tumor environment. Despite the PD-1 expression by tumor infiltrating T cells, blockade of CD38 rewired the metabolic flux of T cells to maintain elevated levels of NAD+ and Sirt1-Foxo1 mediated improved antitumor functions. Thus metabolic reprogramming of T cells by CD38mAb holds significance in the widespread application of adoptive cell transfer as an immunotherapeutic modality for cancer treatment.
Journal article: Chatterjee et al., 2018. CD38-NAD+Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response. Cell Metabolism
Article by Rushikesh Patil