Brain tumours represent one of the greatest challenges in modern cancer immunotherapy. Unlike other malignancies, they develop within the brain’s immune-privileged environment, a highly protected space designed to limit inflammation and safeguard delicate neural tissue. This protection, while vital for brain health, makes it harder for the immune system, and immunotherapies, to do their job.
Recent research has revealed that it’s not simply the brain’s location that dictates how tumours respond to immunotherapy, but the type of tumour itself (Figure 1). Metastatic brain tumours, those that spread from cancers elsewhere in the body, tend to be more immunogenic, meaning the immune system can recognize and attack them more readily. In contrast, primary brain tumours such as gliomas have evolved sophisticated mechanisms to suppress immune responses and evade detection, making them far less responsive to current treatments.
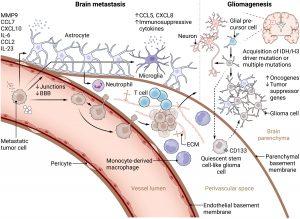
Figure 1: Development of brain metastases and gliomas. Left: Brain metastatic cascade. Circulating tumor cells that have intravasated into the blood vessels secrete soluble factors like chemokines and microRNA-loaded extracellular vesicles, thereby affecting the local CNS microenvironment and inducing premetastatic niche formation. This involves loss of endothelial junctions and BBB integrity, microglial secretion of immunosuppressive cytokines and chemokines, and reprogramming of astrocytes, which secrete cytokines, chemokines, and other soluble factors to recruit tumor cells and facilitate BBB breakdown. Later, reprogrammed astrocytes and microglia promote metastatic niche formation and metastatic outgrowth. Via selectins, tumor cells adhere to the local CNS endothelium and extravasate via transcellular or paracellular routes into the perivascular space between endothelial and parenchymal basement membranes, where they, supported by the ECM, form micrometastases and exploit local blood supply by vessel co-option or angiogenesis before infiltrating the brain parenchyma. Right: Gliomagenesis. In contrast, gliomas originate from glial precursor cells in the brain parenchyma via IDH or H3 driver or multiple mutations and suppression of tumor suppressor and/or activation of oncogenes. They colonize the perivascular space during invasive progression, where they retain a quiescent stem cell–like phenotype before outgrowth. In the parenchyma, glioma cells form functional networks with neurons, astrocytes, and malignant cells to facilitate tumor growth and invasion. At the same time, they render their microenvironment immunosuppressive.
The Two-Fold Challenge for Immunotherapy
Modern immunotherapies, including checkpoint inhibitors, cancer vaccines, and adoptive T-cell therapies, aim to activate and sustain tumour-targeting T cells. But brain tumours pose two critical obstacles:
• Getting T cells into the brain in sufficient numbers.
• Keeping them functional within the tumour’s deeply immunosuppressive microenvironment.
Inside the brain, a network of tumour-associated cells, signalling molecules, and structural barriers can blunt immune activity. As a result, T cells often arrive exhausted, excluded from the tumour core, or rendered ineffective.
To overcome these challenges, researchers are dissecting the cellular and molecular determinants of brain tumour antigenicity, the degree to which tumours are recognized by the immune system. Using single-cell sequencing, spatial profiling, and molecular imaging, scientists are charting how immune cells interact with tumour cells and pinpointing where those interactions fail.
This high-resolution view is helping to identify new strategies to reprogram the tumour microenvironment, such as:
• Enhancing T-cell trafficking across the blood–brain barrier.
• Modulating immunosuppressive signals from tumour-associated macrophages and microglia.
• Combining checkpoint inhibitors with targeted or metabolic therapies to sustain T-cell activation.
Future progress in brain tumour treatment will depend on therapies that both boost immune attack and dismantle local immune suppression. By decoding the molecular roadblocks that limit immune access and persistence in the brain, researchers are paving the way for a new generation of combinatorial immunotherapies.
Brain tumours may hide behind powerful immune barriers, but their unique immune landscape, not just their location, determines how they respond to treatment. Understanding and overcoming these hidden layers of resistance could finally unlock the full potential of immunotherapy for both primary and metastatic brain cancers.
Journal article: Bunse, L., et al. 2025. The immunology of brain tumors. Science Immunology.
Summary by Stefan Botha










