CAR-T cell therapy, a efficient form of cancer immunotherapy, has revolutionised treatment for many patients with aggressive blood cancers. But while the therapy has led to remarkable long-term remissions, some patients report lingering cognitive issues, often described as “brain fog,” including forgetfulness and difficulty concentrating.
A recent study reveals that these cognitive side effects are a real, measurable phenomenon and occur independently of chemotherapy or cancer location (Figure 1). The study also uncovers the cellular mechanisms behind the cognitive impairment and identifies potential therapeutic strategies to reverse it.
The research team used mouse models to explore how CAR-T therapy affects the brain. They tested the treatment in mice with tumors in various tissues including brain, blood, skin, and bone, to determine whether cognitive impairment stemmed from the therapy itself or from secondary immune responses triggered by cancer location.
Surprisingly, mild cognitive impairments occurred even in mice with tumours located completely outside the brain, suggesting that the treatment, rather than the tumour, is the key driver. Only mice with bone tumours, which provoked minimal systemic inflammation, were spared from cognitive changes.
CAR-T therapy-induced cognitive symptoms closely resembled brain fog seen in patients recovering from chemotherapy, radiation, and respiratory infections like influenza or mild COVID-19. This suggests a common biological mechanism across different causes of cognitive dysfunction.
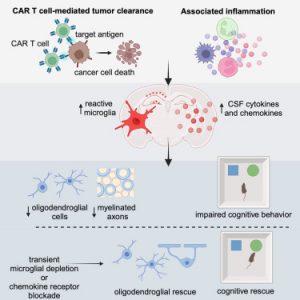
The culprit? The brain’s microglia which are resident immune cells that become “activated” in response to systemic immune signals from CAR-T therapy. Once activated, microglia release inflammatory cytokines and chemokines, which disrupt brain homeostasis. These inflammatory signals particularly damage oligodendrocytes, the cells responsible for producing myelin, a fatty sheath that insulates neurons and supports cognitive function.
Loss of myelin reduces the brain’s ability to transmit signals efficiently, leading to cognitive slowing and memory issues—core features of brain fog.
To confirm their findings, the researchers examined postmortem brain tissue from human patients who participated in a CAR-T trial for brain and spinal tumours. The same patterns of microglial activation and oligodendrocyte disruption were observed.
The team also tested intervention strategies in mice. In one approach, they temporarily depleted microglia from the brain. When the cells repopulated two weeks later, they returned to a healthy, non-inflammatory state and cognitive deficits disappeared.
In a second approach, the scientists administered a compound that blocked chemokine signalling through a specific receptor in the brain. This strategy also rescued cognitive function, suggesting a clinically actionable target for drug development.
As CAR-T therapies continue expanding into more cancer types—including solid tumors and pediatric brain cancers—understanding their neurological side effects is critical for optimizing patient outcomes.
Journal article: Geraghty, A.C., et al. 2025. Immunotherapy-related cognitive impairment after CAR T cell therapy in mice. Cell.
Summary by Stefan Botha