Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infections (LRTIs) in infants, the elderly, and immunocompromised individuals. Despite recent progress, such as the approval of vaccines like Arexvy and Abrysvo, these are limited in their effectiveness, targeting only specific age groups. Given the high global burden of RSV, there is a pressing need for a broad-spectrum vaccine that could target various population groups. This study, published in Frontiers in Immunology, takes a significant step in addressing this gap by using computational methods to identify T-cell epitopes that could lead to a more effective vaccine.
Research Focus and Methodology
The research aimed to identify CD4+ and CD8+ T-cell epitopes from RSV proteins (F, G, and SH) using an immune-informatics approach. The team first screened and predicted the most promising epitopes, followed by experimental validation in female BALB/c mice. The peptides were tested for their ability to elicit immune responses, including antibody production and the activation of key immune cells like lymphocytes.
Of particular interest were the CD8+ T-cell epitope 3RVMHCI and the CD4+ T-cell epitope 4RVMHCII, both from the F protein of RSV. The F protein plays a crucial role in viral fusion with host cells, making it a prime target for vaccine development. These epitopes were selected based on their antigenicity scores and their ability to bind major histocompatibility complex (MHC) molecules, essential for triggering an immune response.
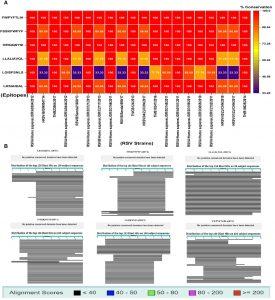
Figure 1 (A) Conservation study of putative vaccination epitopes using 20 pathogenic human RSV strains; (B) non-homologous sequence aliment of epitopes with human proteome.
Key Findings
The study showed that 3RVMHCI (CD8+) and 4RVMHCII (CD4+) induced robust immune responses in the mice models. Following the administration of the second booster dose, 3RVMHCI showed a significant increase in white blood cell count (19.72 × 103 cells/ml) and lymphocyte levels (12.98 × 103 cells/ml). Similarly, 4RVMHCII demonstrated elevated levels of white blood cells (27.08 × 103 cells/ml), neutrophils, and lymphocytes, in addition to high concentrations of IgG antibodies and IFN-gamma. These responses indicate that the epitopes successfully activated both humoral (antibody-mediated) and cellular immune responses.
In-vitro studies further supported these findings, showing that 4RVMHCII produced a significant level of antibodies in the sera of infected mice on day 45, comparable to the natural immune response against RSV. The epitopes also induced high IFN-gamma and IL-2 levels, both of which are critical markers of a strong immune response.
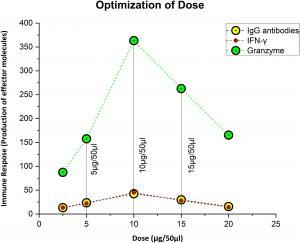
Figure 2 Figuring out the ideal, safe dosage. Ten microgram was thought to be the optimal dose, since it elicited the strongest immunological response, whereas 5 µg was thought to be the priming dose.
Conclusion and Future Implications
This study represents a promising advancement in RSV vaccine development. The identification of these T-cell epitopes opens up new possibilities for creating vaccines that provide broader protection across different age groups. Although the study focused on RSV, the techniques used—particularly the immune-informatics approach—could be applied to develop vaccines against other viruses.
Future research will need to validate these findings in human clinical trials. If successful, this vaccine strategy could significantly reduce the global burden of RSV and provide a blueprint for combating other respiratory viruses.
Summary by Faith Oluwamakinde










