Sepsis occurs when the body’s response to infection spirals out of control, causing organ failure (like the kidneys or lungs) and often leading to death. Historically, scientists believed overactive inflammation was the main culprit. However, more recent findings show that many sepsis patients die because their immune systems become dangerously suppressed—a condition known as immune paralysis. In this state, the body struggles to control existing infections and becomes highly vulnerable to new infections, including fungal invasions.
Sepsis—a life-threatening condition caused by a dysregulated immune response to infection—remains a major challenge worldwide. Scientists have discovered that an existing drug, interferon beta, can rejuvenate immune cells weakened during sepsis (Figure 1). Their study offers promising leads toward future treatments to reverse immune paralysis and improve survival rates.
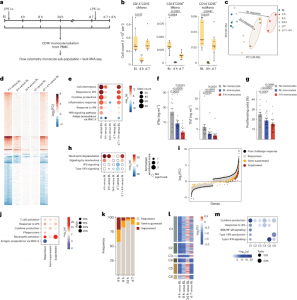
Figure 1: Impairment of IFN-I pathway in blood monocytes 1 week after LPS-induced systemic inflammation. a, Schematic representation of the study and sample acquisition in healthy volunteers (n = 11). Seven volunteers were intravenously (i.v.) injected with LPS (2 ng kg−1) at baseline (BL, d0) and d7. Four volunteers were intravenously injected with placebo (0.9% NaCl) at BL. Peripheral blood was collected at BL, 4 h, 8 h, 24 h, d7 and d7 + 4 h. b, Box plots of absolute abundance of CD14+CD16− cMonos, CD14+CD16+ iMonos and CD14−CD16+ ncMonos in blood of LPS-challenged volunteers (n = 7) at BL, 4 h and d7. c, Principal component analysis of blood CD14+ monocyte transcriptomes from LPS-challenged volunteers (n = 3) at BL, 4 h, 8 h, 24 h, d7 and d7 + 4 h. d, Heatmap representation of DEGs at 4 h, 8 h, 24 h, d7 and d7 + 4 h compared with BL as in c. e, GO term analysis of DEGs at 4 h, 8 h, 24 h, d7 and d7 + 4 h compared with BL as in c. f, Bar plots of TNF and IFNγ production in CD3+ T cells isolated from the blood of healthy donors (n = 9), activated with CD3 and CD28 antibody-coupled beads and co-cultured with cMonos obtained from LPS-challenged volunteers (n = 6) at BL and 4 h at 1:2 ratio. g, Bar plots of percentage of proliferating CD3- and CD28-activated CD3+ T cells isolated from the blood of healthy donors (n = 9), and co-cultured with cMonos obtained from LPS-challenged volunteers (n = 6) at BL and 4 h at 1:2 ratio as in f. h, GSEA of gene expression profiles at 4 h, 8 h, 24 h, d7 and d7 + 4 h compared with BL as in c. i, DEGs in blood CD14+ monocytes from LPS-challenged volunteers (n = 3) in response to both LPS challenges (first: 4 h versus BL; second: 7 d + 4 h versus 7 d) clustered into three groups: responsive (FC < 2), semi-suppressed (2 < FC < 3) and suppressed (FC > 3). j, GO term analysis of responsive, semi-suppressed and suppressed genes in CD14+ monocytes from LPS-challenged volunteers (n = 3) as in j. k, Percentage of responsive (FC < 2), semi-suppressed (2 < FC < 3) and suppressed (FC > 3) DEGs in blood CD14+ monocytes from LPS-challenged volunteers (n = 3) obtained at 4 h, 8 h, 24 h and d7 that were ex vivo stimulated with LPS (10 ng ml−1) versus CD14+ LPS-stimulated monocytes obtained at BL. l, Heatmap representation of average expression of DEGs in CD14+ restimulated monocytes from LPS-challenged volunteers (n = 3) obtained at 4 h, 8 h, 24 h and d7 based on fold-change relative to BL (n = 3) as in k. Genes were clustered (C1–C6) based on their behavior across the different time points. m, GO term analysis of genes in clusters C1–C6 defined as in l. The box plots in b show the median, first and third quartiles and the whiskers 1.5× the interquartile range (IQR). The bar plots in f and g are presented as mean values ± s.e.m. The P values were calculated using two-sided, paired Wilcoxon’s signed-rank tests.
To better understand immune dysfunction in sepsis, the team conducted controlled experiments in healthy volunteers. Participants received endotoxins—harmless fragments of dead bacteria—to simulate a sepsis-like immune response.
Key findings:
- Monocytes, crucial infection-fighting cells, failed to mature properly during the suppressed phase.
- These immature monocytes exhibited reduced functionality, weakening the body’s defense system.
- Detailed lab analyses allowed researchers to map exactly how and when immune paralysis develops.
The team then tested whether interferon beta, a drug currently used to treat multiple sclerosis, could revive these impaired monocytes. In lab experiments:
- Interferon beta stimulated monocyte maturation
- Treated cells recovered their infection-fighting abilities
A treatment that reverses immune paralysis could save thousands of lives every year. With no current therapies that specifically address the suppressed immune response in sepsis, repurposing an existing drug like interferon beta could dramatically accelerate the development of a life-saving intervention.
Journal article: Keramati, F., et al. 2025. Systemic inflammation impairs myelopoiesis and interferon type I responses in humans. Nature Immunology.
Summary by Stefan Botha










