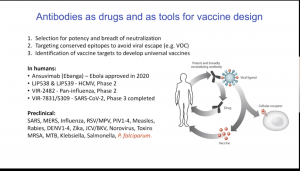
 Today’s IUIS-Immunopaedia-Frontiers Webinar highlights is on a talk by Antonio Lanzavecchia# on his research on malaria antibody immunity. Prof Lanzavecchia began his talk by describing how the isolation of memory B cells can lead to the identification of antibodies that bind to highly conserved regions and have the capacity neutralise pathogens. These antibodies are then used to design therapeutic drugs or reverse engineer vaccine antigens that can induce robust immune responses. He then proceeded to discuss Plasmodium falciparum ( a master of disguise) infection:
Today’s IUIS-Immunopaedia-Frontiers Webinar highlights is on a talk by Antonio Lanzavecchia# on his research on malaria antibody immunity. Prof Lanzavecchia began his talk by describing how the isolation of memory B cells can lead to the identification of antibodies that bind to highly conserved regions and have the capacity neutralise pathogens. These antibodies are then used to design therapeutic drugs or reverse engineer vaccine antigens that can induce robust immune responses. He then proceeded to discuss Plasmodium falciparum ( a master of disguise) infection:
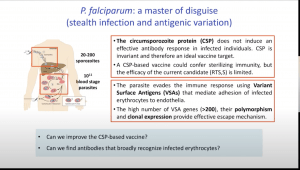 “Plasmodium falciparum uses multiple strategies to evade the human immune response. While infection is established by a small number of sporozoites that are largely ignored by the immune system, the abundant blood stage parasites use multiple and polymorphic variant surface antigens to avoid clearance and subvert the immune response. (source: IUIS Webpage)
“Plasmodium falciparum uses multiple strategies to evade the human immune response. While infection is established by a small number of sporozoites that are largely ignored by the immune system, the abundant blood stage parasites use multiple and polymorphic variant surface antigens to avoid clearance and subvert the immune response. (source: IUIS Webpage)
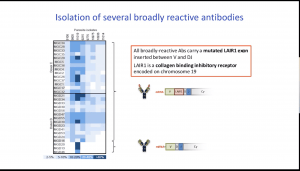 Using a systematic search for antibodies that bind broadly to infected erythrocytes, they discovered, in 10% of malaria-exposed individuals, a new class of antibodies generated by insertions of genomic DNA encoding human inhibitory receptors (LAIR1 or LILRB1) into antibody genes (at the V-DJ junction or in the switch region). LAIR1- (and LILRB1-) containing antibodies bind to different families of parasite RIFINs and opsonize infected erythrocytes. He then described an interesting immunological mechanism whereby antibodies, such as LAIR1 antibodies, that are self-reactive and have high binding affinity for self-proteins undergo somatic hypermutation that leads to the abolishment of self-reactivity a process called clonal redemption.
Using a systematic search for antibodies that bind broadly to infected erythrocytes, they discovered, in 10% of malaria-exposed individuals, a new class of antibodies generated by insertions of genomic DNA encoding human inhibitory receptors (LAIR1 or LILRB1) into antibody genes (at the V-DJ junction or in the switch region). LAIR1- (and LILRB1-) containing antibodies bind to different families of parasite RIFINs and opsonize infected erythrocytes. He then described an interesting immunological mechanism whereby antibodies, such as LAIR1 antibodies, that are self-reactive and have high binding affinity for self-proteins undergo somatic hypermutation that leads to the abolishment of self-reactivity a process called clonal redemption.
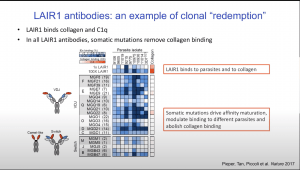 How prevalent are the LAIR1 antibodies specific to P.falciparum antigens? Comparison of participants cohorts from Tanzania, Mali and Europe, demonstrated that approximately 10% of Tanzanian/Malian compared to <1% of Europeans have LAIR1 antibodies. Further clones that dominate antibody immune responses to infected P.falciparum erythrocytes are LAIR1 antibodies. Additionally, he showed that LAIR1 antibodies also have varying configuration of antibodies resulting in bispecific or came-like (lack Kappa and Lambda chains) antibodies. Using these studies he demonstrated how analysing antibody responses to a pathogen can lead to the discovery of antibodies that have unique properties that contribute to pathogen clearance.
How prevalent are the LAIR1 antibodies specific to P.falciparum antigens? Comparison of participants cohorts from Tanzania, Mali and Europe, demonstrated that approximately 10% of Tanzanian/Malian compared to <1% of Europeans have LAIR1 antibodies. Further clones that dominate antibody immune responses to infected P.falciparum erythrocytes are LAIR1 antibodies. Additionally, he showed that LAIR1 antibodies also have varying configuration of antibodies resulting in bispecific or came-like (lack Kappa and Lambda chains) antibodies. Using these studies he demonstrated how analysing antibody responses to a pathogen can lead to the discovery of antibodies that have unique properties that contribute to pathogen clearance.
In summary, these findings demonstrate that the parasite uses multiple RIFINs to target inhibitory receptors as part of its evasion strategy. They also illustrate a new mechanism of diversification based on the insertion of host receptors into immunoglobulin genes, leading to the production of receptor-based antibodies, with implications for antibody and B cell engineering.
Summary by Cheleka Anne-Marie Mpande
Disclosure: this summary contains information made available on the IUIS webinar page










