ARV Drug Classification
 At present, there are four stages during the virus life cycle where ARV drugs can be used:
At present, there are four stages during the virus life cycle where ARV drugs can be used:
- when viral RNA is changed to DNA (reverse transcriptase inhibitor),
- when viral proteins are processed (protease inhibitor),
- when the virus binds to receptors on the human cell (entry inhibitor),
- when viral DNA is incorporated into cellular DNA (integrase inhibitor).
We have included only FDA approved drugs, however, new drugs that target other stages of the virus replication cycle are constantly in development.
Reverse Transcriptase Inhibitors
These drugs interfere with the conversion of viral RNA to DNA by the HIV reverse transcriptase enzyme inside the cell that has been infected.
There are two classes of reverse transcriptase inhibitors:
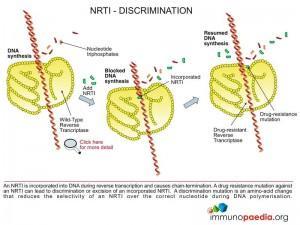 1. Chain terminators (NRTI or NtRTI)
1. Chain terminators (NRTI or NtRTI)
These are drugs that mimic the natural building blocks of DNA (nucleotides), however, once incorporated into the DNA chain by the HIV reverse transcriptase enzyme they do not allow additional nucleotides to be added and are hence named chain terminators or nucleotide base analogues.
Chain terminators can be free of phosphate groups and are referred to as Nucleoside Reverse Transcriptase Inhibitors (NRTI) or they can contain phosphate groups and are referred to as Nucleotide Reverse Transcriptase Inhibitors (written NtRTI). Once inside the cell they require three phosphate groups to be added to generate a nucleotide triphosphate which is the active form of the drug and is used as a substrate for reverse transcriptase during reverse transcription of viral RNA.
Examples of NRTI’s are: AZT or zidovudine, d4T or stavudine, 3TC or lamivudine, FTC or emtricitabine, ddC or zalcitabine, ddI or didanosine, ABC or abacavir.
Example of an NtRTI is: TDF or tenofovir
(Tenofovir has one phosphate group already present and is therefore a nucleotide monophosphate requiring a further two phosphates to be added for activity).
2. Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI)
These are drugs that bind directly to the HIV reverse transcriptase enzyme and prevent it from changing viral RNA to DNA.
Examples of NNRTI’s are: Nevirapine, efavirenz, delavirdine, etravirine.
Download NRTI Guide
Protease Inhibitors (PI)
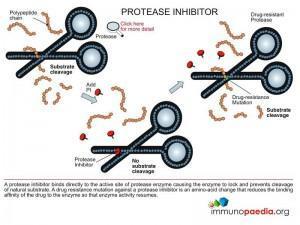 These are drugs that bind to the HIV protease enzyme and prevent it from processing viral proteins.
These are drugs that bind to the HIV protease enzyme and prevent it from processing viral proteins.
Examples of PI’s are: APV or amprenavir, TPV or tipranavir, IDV or indinavir, SQV or saquinavir, RTV or ritonavir, DRV or darunavir, ATV or atazanavir, NFV or nelfinavir
Download Protease Inhibitors Guide
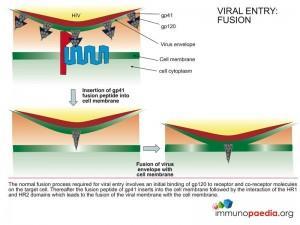 Entry Inhibitors (EI)
Entry Inhibitors (EI)
These drugs prevent entry of the virus into the human cell by interfering with binding of the virus to receptors on the cell surface. These drugs can either target the virus (ie. envelope proteins gp120 and gp41) or the cell receptors (ie. CD4 and CCR5/CXCR4).
There are currently two entry inhibitors that are FDA approved and they fall into two subclasses:
- Fusion inhibitors – Example: Enfuvirtide (binds to HIV gp41)
- CCR5 antagonists – Example: Maraviroc (PDF) (binds to CCR5)
Download Entry Inhibitors Guide
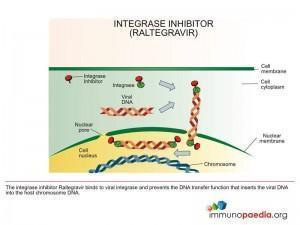 Integrase Inhibitors (II)
Integrase Inhibitors (II)
These are drugs that bind to the HIV integrase enzyme and prevent it from incorporating viral DNA into cellular DNA.
Example of an II is: Raltegravir
Download Integrase Inhibitors – Raltegravir Guide
ARV Drug Summary
Currently approved drugs for the treatment of HIV
Our quick-reference guide has been designed to give you a comparison of all the currently available antiretrovirals for the treatment of HIV. For your interest we have included dosing, side effects, warnings & and any additional considerations such as dietary advice and restrictions. We work consistently to keep this information up to date to ensure you always have access to the latest information.
See www.aidsmeds.com
Download Currently Approved Drugs for HIV
Paediatric Dosing Guidelines
Download Pediatric Dosing Guidelines – Selected ARV’s
ARV Drug Toxicity
 Guiding principles in the management of ARV drug toxicity
Guiding principles in the management of ARV drug toxicity
1. Determine the seriousness of the toxicity.
2. Evaluate concurrent medications and establish whether the toxicity is attributable to an ARV drug or to a non-ARV medication taken at the same time.
3. Consider other disease processes (e.g. viral hepatitis in a child on ARV drugs who develops jaundice) or immune reconstitution syndrome, because not all problems that arise during treatment are caused by ARV drugs.
4. Manage the adverse event according to severity. In general:
- Severe life-threatening reactions: Immediately discontinue all ARV drugs, manage the medical event (i.e. symptomatic and supportive therapy) and reintroduce ARV drugs using a modified regimen (i.e. with an ARV substitution for the offending drug) when the patient is stabilized
- Severe reactions: Substitute the offending drug without stopping ART.
- Moderate reactions: Consider continuation of ART as long as feasible. If the patient does not improve on symptomatic therapy, consider single-drug substitutions. For a few moderate toxicities (e.g. peripheral neuropathy or lipodystrophy) single drug substitution needs to be considered earlier.
- Mild reactions are bothersome but do not require changes in therapy.
5. Stress the maintenance of adherence despite toxicity for mild and moderate reactions.
6. If there is a need to discontinue ART because of life-threatening toxicity, all ARV drugs should be stopped until the patient is stabilized.
Reference: WHO guidelines ANTIRETROVIRAL THERAPY FOR HIV INFECTION IN INFANTS AND CHILDREN: TOWARDS UNIVERSAL ACCESS.










