- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigation
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & answers
- MCQ
Patient Presentation
A 32 year old female presented to the emergency room with a two month history of worsening dyspnea, cough with production of sputum, haemoptysis and extensive weight loss. At the time of presentation she was wasted and in moderate respiratory distress.
History
- Known RVD positive.
- Diagnosed three years previously but not on ARV’s. Lost to follow-up since initial diagnosis.
Past medical history
- RVD positive
Past surgical history
- Nil
Family history
- Nothing of significance
Allergies
- Nil known
Medication
- Nil
Social history
- 6 pack year history of smoking, stopped 3 years ago
- No ethanol use
- No recreational drugs
Differential Diagnosis
- Community acquired pneumonia
- TB
- Pneumocystis
- Kaposi Sarcoma
Examination
ON ADMISSION:
Appearance
- Awake and alert, ill-looking wasted female
Vitals
- Temperature: 38oC
- Blood pressure:110/70
- Heart rate: 100
- Respiratory rate: 36 breaths per minute
General
- Conjunctival pallor
- Cervical, axillary and inguinal lymphadenopathy
ENT
- Raised purple nodule on palate, 4cm in length
Chest
- Crackles bilaterally
Abdomen
- Not distended
- Soft, no generalised tenderness
- No organomegally
- Bowel sounds present
Dermatological
- Two violaceous plaques, non tender, 2cm in diameter on the upper back.
On Admission:
| Reference Ranges | ||
|---|---|---|
| WCC | 5 | 4-12x10 9 /L |
| HB | 8.5 | 12.1-15.2g/L |
| Platelets | 138 | 140-450x10 9 /L |
| CD4+ | 35 cells/mm3 | |
| Arterial Blood Gas | ||
| pH | 7.4 | 7.35 - 7.45 |
| PCO 2 | 33.8 mmHg | 35-45 mmHg |
| PO 2 | 52.2 mmHg | 80 - 100 mm Hg |
| O 2 sats | 89.3 |
Chest X ray:
- Diffuse reticular nodular infiltrate
- Peri-hilar interstitial infiltrates bilaterally
- Left sided pleural effusion
CT Chest:
- Peri-bronchovascular interstitial thickening
- Multiple small nodules in parenchyma of both lungs
- Thickening of interlobular septa
- Hilar lymphadenopathy
- Small left pleural effusion
Sputum smears:
- TB negative X3
Biopsy of lesion on palate
- Macroscopic: Specimen consists of a nodular fragment of mucosa-covered tissue with a blue appearance, measuring 11 x 6 x 4mm
- Microscopic: fragments of squamous mucosa covered tissue showing ectatic, slit-like vascular spaces surrounded by a spindle cell proliferation admixed with plasma cells and extravascated red blood cells. Scattered neutrophils are also seen and hyaline globules are present. The overlying mucosa is reactive.
Features are consistent with Kaposi sarcoma.
Immunohistochemistry:
- HHV8-positive, nuclear dot-like staining.
Open lung biopsy:
- Typical histologic findings of Kaposi sarcoma (as described above)- indicating Pulmonary Kaposi Sarcoma (KS)
Discussion
Introduction
This patient was diagnosed with Kaposi’s sarcoma (KS), which is a spindle cell, angioproliferative, tumour-like lesion that typically develops in the skin, eventually disseminating to multiple cutaneous sites, viscera and lymph nodes. Initially a rare disease, it has increased in prevalence globally because of its association with HIV. In this discussion, we will outline the types of KS, the intrinsic association with human herpes virus type 8 and the immunology behind the development of lesions.
Four clinically relevant and different types of KS have been identified:
- Classical or sporadic KS, originally described as an indolent tumour in the extremities of elderly males of eastern and Mediterranean Europe and it’s not associated with a risk of secondary cancer or an increased mortality rate. It’s most often characterized by isolated cutaneous lesions of the lower limbs (without visceral involvement).
- Endemic KS, predominant in eastern and central subSaharan Africa before the AIDS epidemic and clinically similar to Classical KS, however, lymph node involvement is more frequent and requires systemic treatments as opposed to the classical form and is more fulminant and fatal form in children.
- AIDS associated KS, the most frequent cancer associated with HIV and the most aggressive and rapidly growing form of KS, with early dissemination to the skin and viscera. In recent times its mortality has been dramatically reduced by combined antiretroviral therapy (cART).
- Iatrogenic KS, seen in drug related immunosuppressed patients, e.g. transplant patients. Its presence varies based on the prevalence of HHV-8 in the population.
In spite of the clear clinical differences, all forms of KS are associated with human herpes virus type 8 (HHV-8) also known as Kaposi sarcoma-associated herpesvirus (KSHV). Other diseases associated with KSHV include primary effusion lymphoma or body-cavity-based-lymphoma (PEL/BCBL) found in AIDS patients, Multicentric Castleman’s disease (not discussed here) and lymphedema of the lower extremities, that leads to occlusion of the lymphatic vascular lumen (Dauguet et al., 2023).
This discussion will look at KSHV in immunocompetent patients and in the setting of immunocompromised patients, such as people with HIV, which is likely occurring in our patient. We will also explore the adaptations that make this virus so efficient in circumventing the immune system. Lastly, we will discuss the infection of endothelial cells, with their subsequent transformation and proliferation into spindle cells and the development of KS lesions.
Signs and symptoms of Kaposi Sarcoma
Lesions in KS may involve the skin, oral mucosa, lymph nodes, and visceral organs. Most patients present with cutaneous disease. Visceral disease and lymphedema of the lower limbs may occasionally precede cutaneous manifestations (Dauguet et al., 2023).
Cutaneous lesions in Kaposi sarcoma are characterized as follows:
- Cutaneous lesions may occur at any location but are usually found on the lower extremities, the head and neck region.
- Lesions may have macular, papular, nodular, or plaque like appearances.
- Nearly all lesions are palpable and nonpruritic.
- Lesions may range in size from several millimetres to several centimetres in diameter.
- Lesions may assume a brown, pink, red, or violaceous colour and may be difficult to distinguish in dark-skinned individuals.
- Lesions may be discrete or confluent and typically appear in a linear, symmetrical distribution.
- Mucous membrane involvement is common (palate, gingiva, conjunctiva).
Visceral KS:
KS lesions may also develop in internal organs such as lung and GIT. This disseminated disease is associated with a greater degree of immunosuppression. Signs and symptoms of GIT involvement can include the following:
- Odynophagia, dysphagia
- Nausea, vomiting, abdominal pain
- Hematemesis, haematochezia, melena
- Bowel obstruction
Pulmonary lesions are usually associated with advanced immunosuppression and although they may occasionally be an asymptomatic radiographic finding, appearing as diffuse reticulo-nodules and/or pleural effusion(Cesarman et al., 2019). It is usually associated with evidence of respiratory disease, including:
- Cough
- Dyspnea
- Hemoptysis
- Respiratory failure
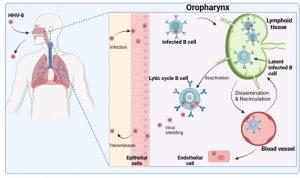
Figure 1 Overview of primary HHV-8 infection. HHV-8 or Kaposi sarcoma-associated herpesvirus (KSHV), is a gamma herpesvirus primarily transmitted through saliva. The virus initially replicates in epithelial cells of the oropharynx and subsequently targets B cells (the primary viral reservoir). Infection of B cells usually results in viral latency characterised by minimal gene expression. These cells are not readily detectable by immune surveillance. Trafficking of infected B cells to lymphoid tissues allows additional B cells to be infected when lytic replication is triggered. Latently infected B cells can disseminate to other parts of the body via the blood. HHV-8 also has the ability to infect endothelial cells potentially causing malignancy. Lytic infection of B cells in the oropharynx may promote infection of epithelial cells and shedding of virus into saliva for transmission to new hosts. Latent infection of B cells are undetectable for long periods of time and the virus is therefore present lifelong. HHV-8 infection is usually asymptomatic when the immune system is intact. Created in https://BioRender.com (CA Petersen 2025)
Overview of primary Human Herpes Virus type 8 (HHV-8) infection
To better understand HHV-8 infection we need to first look at how humans become infected with the virus and the viral replication cycle in the cell. HHV-8 or KSHV is a gamma herpesvirus which is primarily transmitted through saliva. Following infection, the virus establishes a life-long persistent infection in the new host as a result of multiple immune evasion mechanisms. The virus initially replicates in epithelial cells and B cells of the oropharynx and subsequently targets B cells, the primary viral reservoir. Infection by HHV8 results in one of two infection states in the cell. This is depicted in graphic 1. In lytic infection, many viral genes are expressed, the cell makes new virus particles and it dies after a short time period. In latent infection, only a few viral genes are expressed. This limited gene expression enables the infected cell to escape immune surveillance and survive long term.
Latently infected B cells then travel to different areas around the body. Those that traffic to lymphoid tissues may be triggered into the lytic replication cycle by various signals such as inflammation. Lytically infected B cells are thought to promote infection of other cell types such as endothelial cells. Infected hosts will typically remain asymptomatic provided their immune system is intact but may shed virus asymptomatically in saliva from time to time. In our case study, the patient has an immunocompromised immune system, due to HIV infection, and thus her ability to control HHV-8 is poor. HHV-8 infection of endothelial cells is necessary for the development of Kaposi sarcoma.
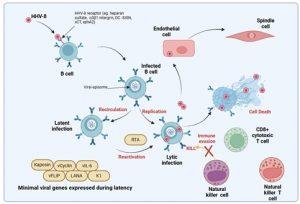
Figure 2 Normal control of HHV-8 infection. HHV-8 can use a variety of receptors to gain entry into target cells that are primarily B cells but also epithelial and endothelial cells. The majority of B cells are latently infected and contain episomal copies of viral DNA in the nucleus which expresses a minimal number of viral genes needed to maintain the viral episomes and prevent activation of the lytic cycle. The lytic cycle is activated by external events such as immune activation of the B cell via BCR stimulation or interaction with CD4+ helper T cells. Latent viral proteins inhibit the activation of the B cell, however, periodic reactivation of infected B cells does occur to promote transmission to other hosts. Additional genes transcribed during lytic replication also interfere with anti-viral immunity involving both innate and adaptive responses. Created in https://BioRender.com (CA Petersen 2025)
Normal immune control of HHV-8 infection
HHV-8 can use alternative receptors, including heparin sulphate, a3b1 integrin and DC-SIGN, to gain entry into target cells. As described, the target cells are primarily B cells but the virus can also infect epithelial, endothelial cells, monocytes and dendritic cells, gaining enrty into the cells via surface receptors such as integrin, cystine-glutamate transporter and tyrosine protein kinase receptor (Cesarman et al., 2019). Once infected, most B cells are latently infected, containing episomal copies of viral DNA which expresses a minimal set of viral genes needed to maintain the viral episomes and prevent activation of the lytic cycle. These minimal viral genes are depicted in graphic 2 as Kaposin, vFLIP, vCyclin, LANA, vIL-6 and K1. The episome is tethered to the host chromosome allowing for viral genome replication during normal cell division.
The lytic cycle may be activated by external events such as immune activation of the B cell via B cell receptor (BCR) stimulation or interaction with CD4+ helper T cells. Latent viral proteins normally inhibit the activation of infected B cells. Viral genes transcribed during both latent and lytic replication cycles subvert innate and adaptive anti-viral immune responses. HHV-8 infection of vascular endothelial cells transforms them and causes them to adopt a spindle cell morphology (through the action of latency associated genes). These spindle cells produce cytokines which cause them to proliferate and under normal conditions this proliferation is well controlled. The process of infection of endothelial cells in an immunocompromised host will be described in the next slides.
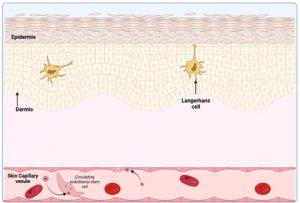
Figure 3 HHV-8 infection of endothelial cells. A common manifestation of HHV-8 infection, when the immune system is compromised, is the development of malignant lesions in the skin, although involvement of mucosal or visceral sites are also possible. It is thought that these lesions develop when vascular endothelial cells in skin capillaries become infected with HHV-8. This may occur by direct infection of capillary endothelial cells or via recruitment of circulating infected endothelial progenitor cells to the vasculature. In immunocompetent individuals, HHV-8 infection is usually asymptomatic, since virus replication is controlled. The virus escapes detection by existing in latent form, however, if HIV infection occurs or immunosuppressive therapy is introduced, reactivation of latent virus is increased leading to increased infection of endothelial cells. Created in https://BioRender.com (CA Petersen 2025)
HHV-8 infection of endothelial cells
When the immune system is compromised a common manifestation of HHV-8 infection is the development of malignant lesions in the skin, although involvement of mucosal or visceral sites are also possible. It is thought that these lesions develop when vascular endothelial cells in skin capillaries become infected with HHV-8. Which occurs by direct infection of capillary endothelial cells or via circulating infected endothelial progenitor cells. In immunocompetent individuals, HHV-8 infection remains asymptomatic because viral replication is controlled and existing virus escapes detection by remaining in latent form. However, if HIV infection occurs or immunosuppressive therapy is introduced, reactivation of latent virus is increased leading to increased infection of endothelial cells.
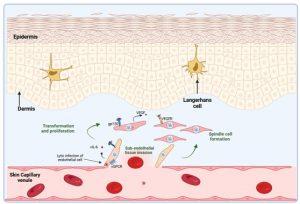
Figure 4 Transformation of HHV-8 infected endothelial cells. Infection of endothelial cells leads to their transformation and proliferation. Invasion of the subendothelial cell layer, such as the dermis of the skin, occurs. Proliferation is mainly driven by cytokine stimulation of latently infected cells in a paracrine manner. Viral cytokines such as vIL-6, a homologue molecule of human IL-6, stimulates the production of VEGF. Viral IL-6, produced by latent and lytically infected cells, is able to stimulate the gp130 chain of the IL-6 receptor and results in modulation of gene transcription. The viral GPCR, a constitutively active homologue of the IL-8 receptor, is expressed in lytically infected cells and also alters intracellular signalling pathways to promote cell transformation and cytokine production. The secretion of VEGF is a primary driver of endothelial cell proliferation via the VEGF receptor. Most of the proliferating cells are latently infected with HHV-8 and develop into characteristic spindle cells. Created in https://BioRender.com (CA Petersen 2025)
Transformation of HHV-8 infected endothelial cells
Transformation of HHV-8 infected endothelial cells
Infection of endothelial cells leads to their transformation and proliferation by virus encoded gene products. Invasion of the subendothelial cell layer, such as the dermis of the skin, occurs. Proliferation of spindle cells is mainly driven by cytokine stimulation. Viral cytokines produced by virus infected cells such as viral (v) IL-6, a homologue molecule of human IL-6, stimulates the production of vascular endothelial growth factor (VEGF). Viral IL-6, is able to bind to the gp130 chain of the human IL-6 receptor, but does not need to bind the co-receptor to exert its activity. Because of this the vIL-6 acts on a wider range of target cells (although it is intrinsically less active than the human protein) (Cesarman et al., 2019; Wen et al., 2022).
The virus also expresses a constitutively active homologue of the IL-8 receptor, known as viral GPCR, which is a key protein in the KS disease process. It is expressed in lytically infected cells. It activates intracellular signaling pathways (in the same cell expressing it) to induce production of a battery of cytokines including: IL-8, IL-6, IL-1b, TNFa, bFGF and VEGF. The secretion of VEGF is a primary driver of endothelial cell proliferation via the VEGF receptor. Most of the proliferating cells are latently infected with HHV-8 and develop into characteristic spindle cells.
Formation of a Kaposi Sarcoma lesion
The formation of a KS lesion is due to the increased proliferation of latentlly infected spindle cells. This is driven by low numbers of HHV-8 infected cells that are in the lytic phase. The cytokines produced by these cells, particularly VEGF and vIL-6 drive the other spindle cells to proliferate (by para-crine oncogenesis). VEGF promotes additional angiogenesis that supplies oxygen and nutrients to the tumour cells. Increased vascularisation and trapping of extravasated red cells in spaces between the spindle cells leads to the characteristic red colour of the lesions. Infiltration of immune cells such as T cells, plasma cells and macrophages, is also a consequence of chemokine release from lytic infected cells. Viral chemokines that are homologues of human CCL1,CCL2 and CCL3 contribute to the chemotaxis of immune cells to the site of the lesion.
A feature of the formation of KS lesions is Paracrine neoplasia:
KS is not a mono-clonal malignancy. Rather, it is the result of polyclonal proliferation of virus infected spindle cells. This process is driven by viral genes, most of which are expressed in lytically infected cells. In an early KS lesion, most of the proliferating spindle cells are latently infected. Occasional lytically infected cells secrete key cytokines that act in a paracrine fashion to drive the surrounding cells to proliferate further, recruit inflammatory cells to the lesion and induce angiogenesis (Cesarman et al., 2019; Dauguet et al., 2023).
HHV-8 dissemination and development of cutaneous lesions
Kaposi sarcoma lesions develop in the skin at late stages of HHV-8 reactivation, following failure of the immune system to control virus replication. Widespread dissemination to the viscera and mucosa may also occur. Thus, when our patient was diagnosed with pulmonary KS, it was indicative of systemic disease and explained her symptoms such as fever, drenching night sweats and weight loss. These cytokine driven responses are always a poor prognostic sign.
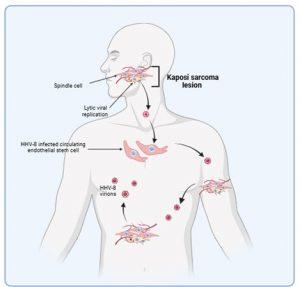
Figure 5 HHV-8 dissemination and development of lesions. Kaposi sarcoma lesions develop in the skin at late stages of HHV-8 reactivation following a failing of the immune system to control virus replication. Dissemination of virus produced by increased lytic replication promotes the development of additional lesions which may also be due to increased infection of circulating endothelial stem cells. Malignancies can also develop at mucosal sites or in other organs. Spindle cells do not appear to metastasise to other sites, rather new lesions develop following free virus infections of endothelial cells or recruitment of infected endothelial cells to the vasculature. Created in https://BioRender.com (CA Petersen 2025)
Treatment
- Following admission this patient was started on empiric TB therapy.
- First-line ARVs were initiated – 3TC, Tenofovir and efavirenz. (If a patient has non visceral disease HAART may be tried as the sole treatment).
- Due to the presence of visceral disease this patient was also referred for chemotherapy: vincristine, doxorubicin and bleomycin.
Although local therapy was not administered to this patient, the following local therapies can be used for locally advanced disease of in patients with cosmeticaly unacceptabe lesions, including:
- Radiation therapy
- Cryotherapy
- Laser therapy
- Surgical excision
- Intralesional vinca alkaloid therapy
- Topical retinoids
Final Outcome
The patient experienced a severe episode of haemoptysis, and died from uncontrolled pulmonary haemorrhage.
Pulmonary involvement is the most common cause of mortality in these patients.
References
Cesarman, E., Damania, B., Krown, S. E., Martin, J., Bower, M., & Whitby, D. (2019). Kaposi sarcoma. Nature Reviews Disease Primers 2019 5:1, 5(1), 1–21.
Dauguet, M., Lebbé, C., & Vignes, S. (2023). Lymphedema and Kaposi sarcoma: A narrative review. JMV-Journal de Médecine Vasculaire, 48(5–6), 181–187.
Wen, K. W., Wang, L., Menke, J. R., & Damania, B. (2022). Cancers associated with human gammaherpesviruses. FEBS Journal, 289(24), 7631–7669.
Pyakurel P et al (2007). KSHV/HHV-8 and HIV infection in Kaposi’s sarcoma development. Infect Agent Cancer. Feb 2;2:4.
Sathish N et al (2011) Evasion and subversion of interferon-mediated antiviral immunity by Kaposi’s sarcoma-associated herpesvirus: an overview., J Virol. Nov;85(21):10934-44.
Dittmer DP et al (2013) Kaposi sarcoma associated herpesvirus pathogenesis (KSHV)-an update., Curr Opin Virol. Jun;3(3):238-44.
Ruocco E et al (2013). Kaposi’s sarcoma: Etiology and pathogenesis, inducing factors, causal associations, and treatments: Facts and controversies.,Clin Dermatol. Jul-Aug;31(4):413-22
Mesri EA et al (2010) Kaposi’s sarcoma and its associated herpesvirus.,Nat Rev Cancer. Oct;10(10):707-19.
Dimaio TA et al (2012) KSHV Induction of Angiogenic and Lymphangiogenic Phenotypes., Front Microbiol. Mar 30;3:102
Montaner S et al (2013) Molecular mechanisms deployed by virally encoded G protein-coupled receptors in human diseases., Annu Rev Pharmacol Toxicol. 53:331-54
Evaluation – Questions & answers
What is the diagnosis?
What is the causative organism associated with Kaposi sarcoma?
What are the target cells for this infection?
In what two forms does the virus exist?
What is the benefit of viral latency?
What is the benefit of the lytic phase?
How do Kaposi Sarcoma lesions form?
Why are Kaposi Sarcoma lesions red in colour?
What is a poor prognostic sign of KS?
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz










