- Patient presentation
- History
- Differential diagnosis
- Examination
- Follow-up Examinations
- Investigations
- Discussion
- Final Outcome
- References
- Evaluation - Questions & answers
- MCQs
Patient Presentation
Jade, 20 years-old, has had episodes of paroxysmal dyspnea since the age of 12, especially at night, associated with coughing and chest tightness, sometimes with wheezing. For 1 year now, attacks have been more frequent, almost daily, also occurring during the day.
Acknowledgement
This case study was provided by Prof. Olivier Boyer (M.D., Ph.D., head of the Department of Immunology and Biotherapy), Dr. Jérémie Martinet (Pharm.D., Ph.D., immunologist) and Dr. Guillaume Mahay (M.D., Ph.D., pulmonologist) from Rouen University Hospital, Normandy University, France. The authors would like to thank Prof. Winfried Pickl (Vienna, Austria) and Dr. Joana Vitte (Marseille, France) for their critical reading of this case study. We are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
History
In the last year, Jade has had six episodes of acute dyspnea that required presentation to emergency room. Three months ago, she started a treatment combining a long-acting bronchodilator and a high-dose inhaled corticosteroid, as well as a short-acting bronchodilator on demand. This treatment provided some improvement between crises. Yet, she continues to have acute episodes of dyspnea several times a week, spontaneously or triggered by exposure to dust or to cat fur. After another severe episode, she decided to consult an allergist.
Past medical history
- Atopic dermatitis between 6 months and 2 years of age
- Clear rhinitis all year round, especially in the morning when waking up
- Menstruating since the age of 12
Surgical history
- Appendix removed at 8 years of age
Family history
- Her mother is asthmatic
- Her younger brother has atopic dermatitis
Travel history
- None
Social history
- Law student
- No active or passive smoking
- Mother’s occupation: teacher
- Father’s profession: administrative agent
Medication
- Inhaled combination of Fluticasone 500 µg and Salmeterol 50 µg twice daily
- Salbutamol on demand
- Oral contraception
Differential diagnosis
- Allergic asthma
- Allergic bronchopulmonary aspergillosis
- Non allergic asthma
- Granulomatosis with polyangiitis
- Cystic fibrosis
- Chronic obstructive bronchopneumopathy
Examination
Vitals
- Heart rate: 65/min
- Blood pressure: 117/78 mmHg
- Temperature: 37.2°C
- Oxygen saturation: 98%
- Respiratory rate: 18/min
General
- Weight: 65 kg
- Height: 165 cm
- General condition preserved
- Coughing
Cardiovascular
- Normal heart sounds
- All pulses present
Respiratory
- Symmetrical vesicular murmur
- No added noise
Abdomen
- Normal
Neurological
- Osteotendinous reflexes present and normal
- Examination of the cranial pairs normal
- No sensory or motor deficit
- Normal consciousness
Cutaneous
- Dry skin without other abnormalities
Follow-up clinical examinations
The allergist decides to switch her background treatment to a fixed inhaled combination of high daily doses of corticosteroids and Formoterol, plus an anti-leukotriene and a long-acting inhaled anticholinergic. To treat her allergic rhinitis, an antihistamine is prescribed together with a corticosteroid nasal spray. Jade is also prescribed sessions of therapeutic education with a written action plan in order to optimize her compliance to treatment.
Despite these progressive therapeutic adaptations, Jade reports that her condition is persistent and that she has had to present to the emergency room twice in the last 3 months
Investigations
Values of Laboratory Tests
| Test | Value | Reference range |
|---|---|---|
| WBC | 7.41 | (4-12 x 109/L) |
| HB | 13.8 | (12.1-15.2 g/L) |
| Platelets | 152 | (140-450 x 109/L) |
| CRP | 5 | (0-8 mg/l) |
| Na | 137 | (135-147 mmol/L) |
| K | 4.2 | (3.3-5.0 mmol/L) |
| Cl | 101 | (99-103 μmol/L) |
| Urea | 6.2 | (2.5-6.4 mmol/L) |
| Creatinine | 68 | (62-115 mmol/L) |
| CK | 183 | (25-195 IU/L) |
| Total protein | 72 | (60-80 g/L) |
| Albumin | 42 | (35-50 g/L) |
| Corrected calcium | 2.2 | (2.1-2.6 mmol/L) |
| Immunoglobulins (IgG) | 9.2 | (7-15 g/L) |
| Immunoglobulins (IgA) | 3.1 | (1-4 g/L) |
| Immunoglobulins (IgM) | 1.1 | (0.5-2 g/L) |
| Phosphate | 1.4 | (1.0-1.5 mmol/L) |
| Magnesium | 1.1 | (0.8-1.3 mmol/L) |
| Thyroid stimulating hormone | 11.3 | (9-30 mIU/L) |
| Free T4 | 14.2 | (10-26 pmol/L) |
| Anti-neutrophil cytoplasmic antibodies | negative | negative |
| Aspergillus fumigatus serology (IgG) | negative | negative |
| Immunoglobulins E (total IgE) | 5.5 | |
| Cat dander serology (IgE) | 25.3 | |
| Dermatophagoides pteronyssinus serology (IgE) | > 100 | |
| Dermatophagoides farinae serology (IgE) | > 100 | |
| Alternaria alternata serology(IgE) | 1.2 |
Asthma Control Questionnaire Score: 3.6 (0=no impairment, 6= maximum impairment)
Prick Tests
- Cat dander, positive
- Dermatophagoides pteronissinus, positive
- Dermatophagoides farinae, positive
- Alternaria alternata, positive
Home visit by an indoor environment counsellor
- Lives in a recent house
- No molds
- Mite avoidance measures respected
- No pets
Chest X-ray
- Normal
Chest CT
- Thickened bronchial walls with no other abnormalities
Pulmonary functional testing
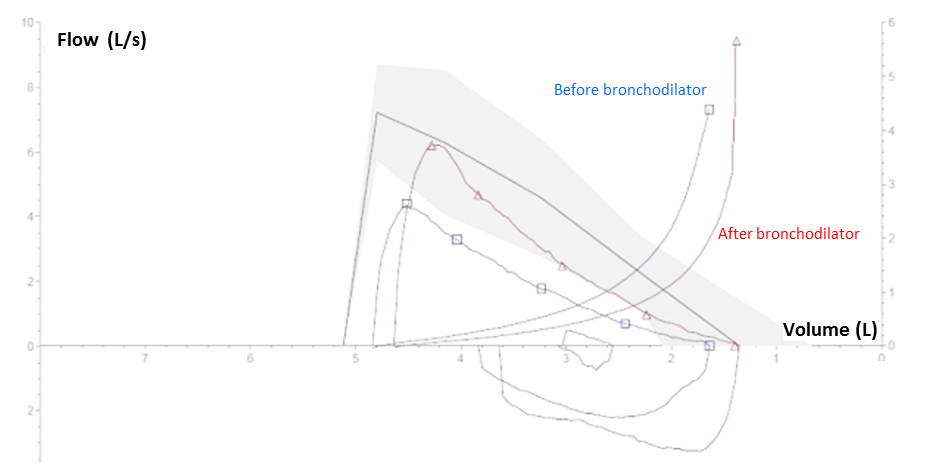
Figure 1. Pulmonary Functional Testing: The values of the curve below and above the x-axis represents inspiration and expiration of air. The solid line corresponds to the flow/volume measure of the patient overtime. The shaded area corresponds to the normal range around the average theoretical values.
| Base | Predicted | %Predicted | Post bronchodilatator | |
|---|---|---|---|---|
| FEV1 (L) | 2.21 | 3.29 | 67 | 2.73 |
| FVC (L) | 3.19 | 3.77 | 85 | 3.25 |
| FEV1/FVC (%) | 69 | 84 | 82 | 78 |
| PEF (L/s) | 4.40 | 7.22 | 61 | 6.20 |
| TLC (L) | 4.89 | 5.10 | 96 | - |
| FRC (L) | 2.83 | 2.72 | - | |
| RV (L) | 1.64 | 1.39 | 118 | - |
FEV1: forced expiratory volume in the first second
FVC: forced vital capacity
PEF: peak exploratory flow
TLC: total lung capacity
FRC: functional residual capacity
RV: residual volume
Pulmonary functional testing (PFT) evidences an obstructive airway syndrome since the FEV1 / FVC ratio is less than 70% (i.e., 69%). This obstructive airway syndrome is reversible as the FEV1 has improved by more than 200 ml (i.e., 2.73 – 2.21 = 0.52 L) and 12% (i.e., 0.52 / 2.21 = 24%) after bronchodilator, confirming the diagnosis of asthma. As the post bronchodilator FEV1 is greater than 80% of predicted value (i.e., 2.73 / 3.29 = 83%) and the FEV1 / FVC ratio becomes greater than 70% (i.e., 78%), the obstructive airway syndrome is completely reversible.
TLC is normal, so there is no restrictive syndrome or chest distention.
Discussion
- Jade’s medical history of asthma
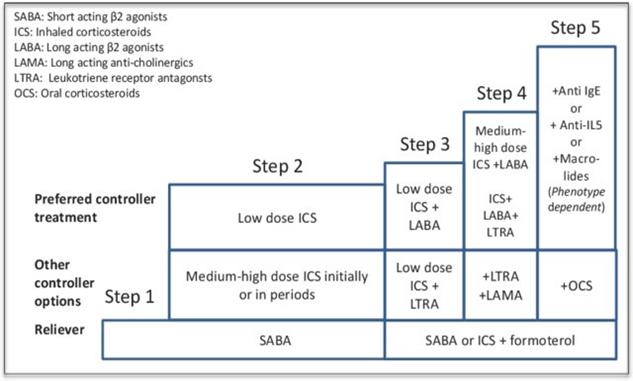
Jade has an atopic background with a family history, a personal history of atopic dermatitis and allergic rhinitis. Her asthma symptoms are triggered by exposure to identified allergens to which she is sensitized. Therefore, Jade has asthma with type 2 allergic inflammation. Despite optimal observance, Jade’s condition remains poorly controlled and she experiences exacerbations of dyspnea. Additional treatments that have not improved her condition after several months have had to be discontinued. Jade’s treatment has been progressively increased according to international recommendations (Global Initiative for Asthma; GINA steps; Figure 1)1 with the prescription of a combined allergic ground and symptom treatment as advised. Therefore, Jade’s diagnosis is severe asthma according to the ERS/ATS definition2, justifying a biologic therapy such as Omalizumab.
The long-acting anticholinergic and antileukotriene treatment was discontinued as it had not led to any improvement.
Jade fulfils the three criteria required for treatment with the anti-IgE monoclonal antibody omalizumab, i.e., severe allergic asthma, resistant to therapy, high IgE level. Accordingly, omalizumab was administered by intravenous route, every 3 months, at a dosage adapted to weight and total IgE levels.
Definition of severe asthma for patients aged ≥6 years (ERS/ATS recommendation)
Asthma which requires treatment with guidelines suggested medications for GINA steps 4–5 asthma (high dose ICS# and LABA or leukotriene modifier/theophylline) for the previous year or systemic CS for ≥50% of the previous year to prevent it from becoming “uncontrolled” or which remains “uncontrolled“ despite this therapy
- Uncontrolled asthma defined as at least one of the following:
1) Poor symptom control: ACQ consistently ≥1.5, ACT<20 (or “not well controlled” by NAEPP/GINA guidelines)
2) Frequent severe exacerbations: two or more bursts of systemic CS (≥3 days each) in the previous year
3) Serious exacerbations: at least one hospitalisation, ICU stay or mechanical ventilation in the previous year
4) Airflow limitation: after appropriate bronchodilator withhold FEV1 <80% predicted (in the face of reduced FEV1/FVC defined as less than the lower limit of normal)
Controlled asthma that worsens on tapering of these high doses of ICS or systemic CS (or additional biologics)
ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; NAEPP National Asthma Education and Prevention Program.
- Pathophysiology of type I hypersensitivity
Jade has typical symptoms of poorly controlled allergic asthma. The diagnosis of asthma is attested by the existence of a reversible obstructive syndrome on pulmonary function test (PFT). Asthma is a chronic disease characterized by shortness of breath, chest tightness or pain, coughing and trouble sleeping due to coughing or wheezing. These symptoms are due to an inflammation of the airways in the lungs which affects the sensitivity of the nerve endings in the airways so they become easily irritated. During an asthma attack, the lining of the airways swells, causing the airways to narrow and reducing the flow of air in and out of the lungs. In allergic asthma, this chronic inflammation is caused by type I hypersensitivity against aeroallergens.
Allergens are antigens, that are either protein in nature or non-proteinaceous haptens bound to a protein carrier which induce type I hypersensitivity3. Type I hypersensitivity reactions are taken to be very immediate and “exaggerated” immune responses mediated by IgE (Abbas, Moussa and Akel, 2022). Aeroallergens are airborne allergens derived from pollen, animal dander, house dust mite and molds. Other more dangerous allergens include insect venom (Abbas, Moussa and Akel, 2022).. The response to the antigen can have two phases;
- The sensitization stage, where the host will have exposure to the antigen without any symptoms occuring.
- The effect stage: The host is re-exposed to the same antigen and this is followed by a type I anaphylactic or atopic immune response, eventually leading to events classified as an allergy .
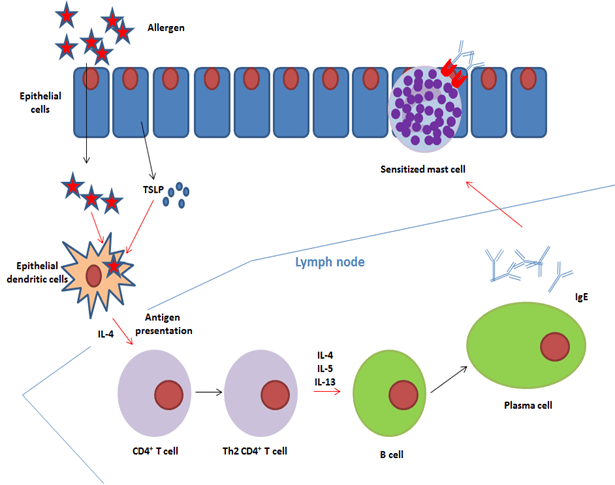
Epithelial dendritic cells activated by thymic stromal lymphopoietin (TSLP) capture, process and transport allergens to the draining lymph nodes4,5. During this process, they differentiate into interleukin-4 (IL-4)-expressing dendritic cells. In response to antigenic presentation by these dendritic cells, naïve T CD4+ cells differentiate into (1) follicular helper T cells that produce IL-4 and (2) Th2 lymphocytes that also produce type 2 cytokines (IL-4, IL-5, IL-13)6. Upon interaction with these pro-allergenic helper T cells, allergen–specific B cells undergo Ig isotype switching in the presence of IL-4 +/- IL-13, and become plasma cells that produce IgE rather than IgG (figure 2)
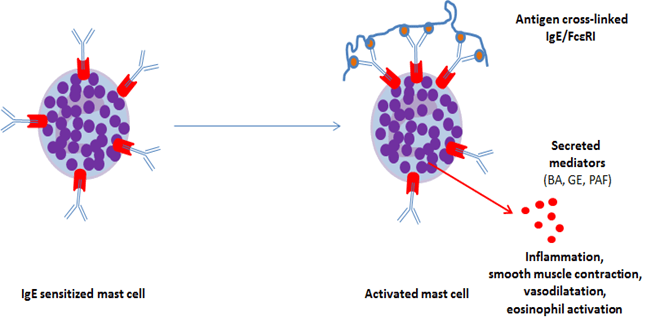
IgE bind to the high affinity FcεRI receptor constitutively expressed on mast cells and basophils. Thus, these cells become sensitized to the allergen. An inducible, incomplete form of FcεRI receptor can be expressed by other cell types, including eosinophils. In case of allergenic re-exposure, bridging of FcεRI occurs upon binding of the multivalent allergen to the IgE on basophils or mast cells, which triggers the immediate release of preformed mediators (biogenic amines, granule enzymes, platelet-activating factor, etc.). In a matter of seconds, this process leads to the production and subsequent release of leukotrienes, prostaglandin D2 and inflammatory cytokines, and eventually to the clinical allergic reaction7 (figure 3).
Histamine is the major biogenic amine released by mast cells and basophils. Its action is time-limited due to the presence of amine-specific transport systems that rapidly clears them from the circulation (half-life 60 seconds). On endothelial cells, histamine causes an increase in inter-endothelial spaces by cell contraction and leakage of plasma into tissue causing edema. It also induces vasodilatation on small vessels. Histamine induces smooth muscle contraction, causing bronchoconstriction in asthma.
Tryptase is the major granule enzyme, preformed and packed in the same secretory granules as histamine, and released during mast cell degranulation. Tryptase degrades fibrinogen in vitro and activates collagenase, potentially contributing to tissue damage. Its proinflammatory effects contribute to the late phase of the allergic reaction. However, its functions in vivo are still under investigation8.
Lipid mediators such as prostaglandin D2 or leukotrienes are synthesized from arachidonic acid in the first hour after mast cell activation and induce vasodilatation and bronchoconstriction.
Finally, activated mast cells and basophils initiate de novo transcription and synthesis of inflammatory cytokines such as IL-1, IL-4, IL-5, IL-6, IL-13 or TNF. These cytokines maintain an inflammatory condition in the late phase of the reaction and further support a pro-Th2 environment.
- Mechanism of action of Omalizumab
Omalizumab is a recombinant humanized IgG1k monoclonal antibody (molecular weight 149 kD) that selectively targets free human IgE and currently used to treat allergic asthma and chronic urticaria (Kumar and Zito, 2022). Omalizumab binds to a site in the C-epsilon-3 (Cε3) domain of each IgE heavy chain that is also involved in IgE binding to the high (FcεRI) and low (CD23) affinity receptors. This therapeutic antibody is produced by modified Chinese Hamster Ovary (CHO) cells.
Omalizumab inhibits the binding of free IgE to the high affinity IgE receptor FcεRI on the surface of mast cells, basophils and eosinophils, thereby putting a pause on the immune events that lead to an allergic reaction. Less FcεRI-bound IgE attenuates release of allergy mediators9, prevents subsequent immune cell activation and eventually induces downregulation of FcεRI receptors through a negative feedback loop. Moreover, omalizumab also inhibits the binding of IgE to the low affinity IgE receptor (CD23) and thereby reduces IgE-facilitated allergen presentation, which, in the long term also reduces IgE titers10. Therefore, omalizumab reduces the inflammatory response to allergen exposure and its long-term consequences11,12.
Due to its mode of action, omalizumab initially tends to cause an increase in serum IgE levels. This is not only based on the reduced binding of IgE to FcERI, but also due to the stabilization of serum IgE by omalizumab binding. What is important in all this is that in highly sensitized individuals the therapeutic benefit of omalizumab treatment becomes apparent only after several weeks of therapy, and then when IgE on effector cells or even whole effector cells are replaced by new ones. Therefore, it is not unexpected for no clinical improvement to be observed in the first weeks of omalizumab treatment (apart from an apparently “paradoxical” increase in serum IgE levels).
Omalizumab is indicated in severe persistent allergic asthma with elevated total IgE. It is also indicated in the treatment of spontaneous chronic urticaria with elevated total IgE. Omalizumab, however, is taken to have different mechanisms of actions for treating both conditions(Kumar and Zito, 2022). Clinical trials and real-life experience have shown the good efficacy of this treatment and the low level of side effects.
Before the initiation of a treatment with omalizumab, a titration of serum total IgE is needed in order to calculate the dose to be injected into the patient according to her/his weight as well determine the frequency at which the medication is administered.
Currently, total IgE assays are not recommended to monitor treatment. Indeed, omalizumab binds to free IgE and may alter the concentration of total IgE by two opposite effects:
- they tend to augment free serum IgE that can no longer bind to their receptor,
- they tend to lower free IgE levels by augmenting the elimination of IgE/omalizumab immune complexes.
Furthermore, currently used routine IgE assays do not allow to differentiate between free and omalizumab-bound IgE.
Due to the possibility of anaphylaxis, patients on this medication are required to be under observation for a suitable period of time after taking this medication (Kumar and Zito, 2022).
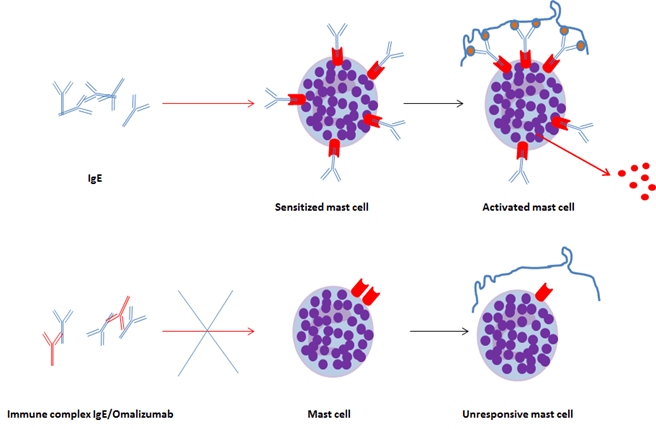
Figure 5. Mechanism of action of Omalizumab
(IgE are depicted as blue Y-shaped antibodies, omalizumab are displayed as red Y-shaped antibodies)
Download images for this case
Final Outcome
During the first 2 months of treatment with omalizumab, no clinical benefit was noted regarding the frequency or the severity of asthma episodes. Since the third month, Jade started to feel gradually better and, after 4 months of biologic therapy, she has experienced no more episodes of dyspnea. Her ACQ score normalized to 0.23 and her pulmonary function tests significantly improved. Because of the excellent clinical response, Jade’s treatment with omalizumab will be maintained in the long term.
Download images for this case
References
- Abbas, M., Moussa, M. and Akel, H. (2022) “Type I Hypersensitivity Reaction,” in StatPearls [Internet]. StatPearls Publishing.
- Hammad, H. and Lambrecht, B. N. (2021) “The basic immunology of asthma,” Cell, 184(6), pp. 1469–1485. doi: 10.1016/j.cell.2021.02.016
- Kumar, C. and Zito, P. (2022) “Omalizumab,” StatPearls. Available at: https://www.statpearls.com/ArticleLibrary/viewarticle/26162 (Accessed: November 25, 2022).
- Thomson, N. C. and Chaudhuri, R. (2012) “Omalizumab: clinical use for the management of asthma,” Clinical medicine insights. Circulatory, respiratory and pulmonary medicine, 6, pp. 27–40. doi: 10.4137/CCRPM.S7793.
- Global strategy for asthma management and prevention. 2020. (Accessed 20/12/2020, at www.ginasthma.org.)
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
Link to Abstract - Scheurer S, Toda M, Vieths S. What makes an allergen? Clin Exp Allergy. 2015;45(7):1150-1161.
Link to Abstract - Schaumann F, Muller M, Braun A, et al. Endotoxin augments myeloid dendritic cell influx into the airways in patients with allergic asthma. Am J Respir Crit Care Med. 2008;177(12):1307-1313.
Link to Abstract - Bratke K, Lommatzsch M, Julius P, et al. Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax. 2007;62(2):168-175.
Link to Abstract - Akdis M, Trautmann A, Klunker S, et al. T helper (Th) 2 predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J. 2003;17(9):1026-1035.
Link to Abstract - Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol. 2014;5:569.
Link to Abstract - Vitte J. Human mast cell tryptase in biology and medicine. Mol Immunol. 2015;63(1):18-24.
Link to Abstract - MacGlashan DW, Jr., Bochner BS, Adelman DC, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158(3):1438-1445.
Link to Abstract - Selb R, Eckl-Dorna J, Twaroch TE, et al. Critical and direct involvement of the CD23 stalk region in IgE binding. J Allergy Clin Immunol. 2017;139(1):281-289 e285.
Link to Abstract - Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115(3):459-465.
Link to Abstract - Djukanovic R, Wilson SJ, Kraft M, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170(6):583-593.
Link to Abstract
Download images for this case
Evaluation – Questions & answers
What is the main cytokine responsible for IgE switching in B cells?
interleukin-4 (IL-4)
What are the main effects of histamine in asthma?
Histamine induces contraction of bronchial smooth muscle, causing bronchoconstriction. Histamine also causes vasodilation of small vessels, endothelial cell contraction, increase in inter-endothelial spaces and leakage of plasmatic fluid into tissues causing edema, aggravating bronchial obstruction.
Explain how mast cells are activated by allergens
Allergen-specific IgE bind to the high affinity FcεRI receptor on mast cells that in turn become sensitized. In case of allergenic re-exposure, bridging of FcεRI occurs upon binding of the multivalent allergen to the IgE on mast cells, which triggers immediate release of preformed mediators (biogenic amines, granule enzymes, platelet-activating factor), the synthesis and release of leukotrienes, prostaglandin D2 and inflammatory cytokines (TNF, IL-1, IL-4, IL-5, IL-6, IL-13, etc.), and eventually a clinical allergic reaction.
What is the main mechanism of action of omalizumab?
Omalizumab is a humanized IgG1k monoclonal antibody that selectively targets free human IgE. Omalizumab inhibits the binding of free IgE to the high affinity IgE receptor FcεRI of mast cells, basophils and eosinophils and also to the low affinity IgE receptor CD23.
Why are total IgE immunoassays not recommended for monitoring the biological efficacy of omalizumab in patients under therapy?
Omalizumab prevents the effector function of allergen-specific IgE on basophils and mast cells, but does not reduce IgE production by B cell-derived plasma cells. Furthermore, current routine total IgE assays do not differentiate between free IgE and IgE complexed to omalizumab. The results of such assays may therefore be misleading.
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz