A myofibroblast population characterized by leucine-rich-repeat-containing protein 15 (LRRC15) has been identified from single cell studies on cancer that have been carried out in both humans and mice. What is yet to be understood better are the molecular mechanisms that give rise to LRRC15+ cancer-associated fibroblasts (CAFs) and how they could potentially affect anti-tumour immunity.
Krishnamurty and colleagues decided to use of mouse model of pancreatic cancer to try and address this. They were able to show that the development of LRRC15+ myofibroblasts during the process of tumorigenesis is supported by TGFβ signalling in dermatopontin positive (DPT+) universal fibroblasts.
Depletion of just CAFs that were LRRC15+ led to a reduction in total fibroblast content (figure 1) and shifted the stromal compartment to a more fibroblast-like state. Regarding immune responses, this boosted CD8+ T cell functions and enhanced anti-PDL1 responsiveness.
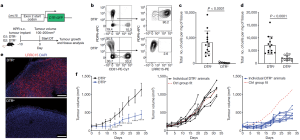
Figure 1: “Targeted depletion of LRRC15+ CAFs significantly reduces tumour growth. a, Schematic of the genetic (top) and experimental (bottom) approach for Lrrc15DTRGFP mice. b–e, Data are from subcutaneous KPR tumours 8 days after DT treatment in DTR– and DTR+ mice. b, Representative flow cytometry plots showing the frequency of PDPN+LRRC15+ cells. Cells were gated on CD24– CD45– cells (left) or PDPN+CD31– cells (right). c,d, Quantification of the total number of PDPN+LRRC15+ cells (c) and PDPN+CD31– cells (d) normalized by tumour weight (n = 12 or 14 mice). e, Representative immunofluorescence images of LRRC15 and DAPI. Scale bar, 250 μm. f, Tumour growth curves from DTR– and DTR+ mice treated with DT (n = 9 or 11 mice per group). Left: average tumour volume across all animals (*P = 0.015, ***P = 0.0006, ****P < 0.0001). Middle and right: individual animal growth curves per genotype. The x axis represents days after DT treatment. The dashed red line represents the average reference fit for the control (Ctrl) group. Data in c and d are pooled from four independent experiments. Data in e and f are representative of two or three independent experiments. Data in c, d and f are the mean ± s.d. Statistics were calculated using two-tailed, unpaired Student’s t-test (c and d) or ordinary two-way analysis of variance (f)”.
A further look into tumours that were depleted of LRRC15+ CAF showed that without the constant removal or depletion, LRRC15+ myofibroblasts were able to constantly replace themselves, grow and multiply in number such that they re-establish their foothold in the CAF compartment. This would show that there is a bit of a ‘back and forth’ relationship between universal fibroblasts and LRRC15+ CAFs which at the end of the day will bring down anti-tumour T cell immunity and reduce the effectiveness of immune checkpoint blockade (ICB) therapy.
The authors had this to say about the overall impact of the work carried out here and the results generated; “Therapeutically, our findings raise the issue of the optimal strategy to modulate LRRC15+ CAF activity. The use of TGFβ inhibitors, which are currently being evaluated in multiple clinical trials, is an attractive therapeutic option to impair LRRC15+ CAF formation. If the optimal environment for an effective anti-tumour immune response requires a CAF compartment that is both smaller and devoid of LRRC15+ CAFs, our data strongly suggest that depleting LRRC15+ CAFs themselves may be a more attractive therapeutic strategy to generate robust and durable responses to cancer immunotherapy”.
Journal article: Krishnamurty, A.T., et al., 2022. LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature.
Summary by Vanessa Muwanga










