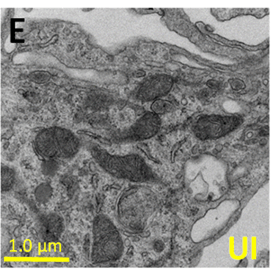
In parenthesis is the % of HIV infected cells. (E,F) correspond to representative pictures of transmission electron microscopy (TEM) of macrophages in control conditions (uninfected, UI) and HIV infected cells (HIV) to observe the distribution, numbers, and size of mitochondria. (Source: Castellano et al., 2019)
Majority of HIV infected macrophages die but the few that survive make up the small population of cells that remain latent. These cells have been found to have a unique metabolic profile that is distinct from other HIV reservoirs. By analyzing the metabolic profile of these cells, Castellano et al., were able to determine factors that may be associated with their survival. The authors suggest that targeting these factors can contribute to the development of therapies that have the ability to eliminate HIV reservoir in macrophages.
HIV infection in macrophages was shown to decrease basal oxygen consumption without affecting functional responses nor changes in oxidative phosphorylation. This was assessed by performing Western blot and RT-qPCR that revealed no change in the protein and gene expression of mitochondrial complexes I to V at different stages of HIV infection. Unlike T-cell viral reservoirs that show elevated levels of glycolysis, this process remains unchanged in macrophages and is therefore not unaffected by HIV infection. HIV also causes the mitochondria to enlarge and these are the areas where lipid droplets tend to aggregate. These lipids are taken to be an additional source of energy for these cells, along with glutamine.
HIV-infected macrophages were able to effectively shift from one energy source to another in comparison to uninfected macrophages. These cells were also found to majorly utilize glutamine as a source of energy unlike other normal cells that use glucose and fatty acids. This kind of metabolic change has also been identified in different cell types that are infected with cancer.
The authors demonstrated the importance of glutamine and glutamate to the survival of these latently-infected macrophages by showing that there was a decrease in the number of cells that survived when these metabolites were blocked. The use of glutamate as a source of energy by these cells, gives enables them to survive independently of efficient blood circulation, the same way that cancer cells do.
The authors suggest that the inhibition of any pathways that heavily depend on the use of glutamine can be targeted and inhibited in order to reduce or even prevent the development of HIV reservoirs. This approach has been used to induce the death of cancer cells and reduce tumor growth for different types of cancers like glioblastoma.
Journal Article: Castellano et al., 2019, HIV infection and latency induce a unique metabolic signature in human macrophages. Nature Scientific Reports.
Article by Vanessa Mwebaza Muwanga











