A fresh perspective on Alzheimer’s disease has led to a promising discovery that could pave the way for preventing the cognitive decline characteristic of this and other neurodegenerative disorders. Researchers have uncovered evidence implicating the immune system—specifically, a molecule called STING—in driving the brain inflammation and damage seen in Alzheimer’s (Figure 1).
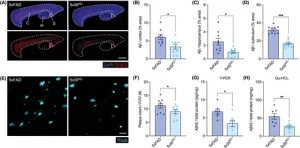
Figure 1: Amyloid beta load is reduced with STING deletion in 5xFAD mice. 5xFAD Sting1−/− (abbreviated as 5xStKO) mice and littermate 5xFAD Sting1+/+ (abbreviated as 5xFAD) controls were harvested at 5 months of age to evaluate Aβ load by IF imaging. (A–D) Analysis of total Aβ burden in the cortex, hippocampus, and subiculum (n = 9 mice/group, six to nine images/mouse, two independent experiments). (A) Representative confocal images of brain sections stained for nuclei (DAPI, blue) and total Aβ (D54D2, red; scale bar = 40 µm) in the cortex (abbreviated as ‘C’), subiculum (abbreviated as ‘S’), and hippocampus (abbreviated as ‘H’). Quantification of percent area covered by D54D2 staining in the cortex (B), hippocampus (C), and subiculum (D). (E,F) Analysis of cortical Aβ plaque burden (n = 9, 9–12 images/mouse, three independent experiments). (E) Representative confocal images of brain sections stained for Aβ plaques (ThioS, cyan; scale bar = 30 µm). (F) Quantification of plaques per FOV. (G,H) Analysis of Aβ42 protein concentrations from whole-brain lysates (n = 13–15 mice/group, three independent experiments). (G) ELISA quantification of Aβ42 per total protein from the soluble fraction extracted with T-PER and (H) the insoluble fraction extracted with Gu-HCL. Statistical significance between experimental groups was calculated by linear mixed-effects models (B–D,F) and unpaired Student’s t test (G,H). Data points represent individual mice and error bars represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Aβ, amyloid beta; DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay; FOV, field of view; IF, immunofluorescence; SEM, standard error of the mean; STING, stimulator of interferon genes.
STING (Stimulator of Interferon Genes) is a key component of the brain’s innate immune defence. Under normal circumstances, it plays a vital role in clearing viruses and damaged cells by responding to DNA abnormalities. However, the team found that when STING becomes overactive in the aging brain, it may inadvertently promote the formation of amyloid plaques and tau tangles—hallmarks of Alzheimer’s pathology.
In experimental models of the disease, inhibiting STING protected mice from cognitive decline. It also reduced microglial activation—an indicator of neuroinflammation—near amyloid plaques and preserved the health of nearby neurons. These findings suggest that STING overactivation fuels damaging immune responses in the brain, accelerating neurodegeneration and memory loss.
The implications extend beyond Alzheimer’s. Because STING is involved in broader immune regulation, it may also contribute to other neurological conditions like Parkinson’s disease, ALS, and various forms of dementia. As such, it represents an attractive therapeutic target for a range of neurodegenerative diseases.
What makes STING particularly noteworthy is its influence over both amyloid and tau pathology, positioning it as a potentially more comprehensive intervention point than previously studied molecules, which often act only during specific phases of disease progression.
This work signals a significant step toward uncovering safer, more effective treatments to protect the aging brain.
Journal article: Thanos, J. M., et al. 2025. STING deletion protects against amyloid β–induced Alzheimer’s disease pathogenesis. Alzheimer’s & Dementia.
Summary by Stefan Botha










