In a new first-in-human trial, researchers have successfully engineered patients’ own blood-forming stem cells to continuously produce cancer-killing T cells, a major step forward in long-lasting immunotherapy for hard-to-treat cancers (Figure 1).
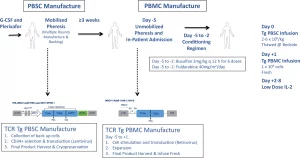
Figure 1: Overview of manufacturing protocol, and clinical trial procedures. Peripheral blood stem cells (PBSCs) were isolated from patients via leukapheresis following bone marrow mobilization with filgrastim and plerixafor. CD34+ cells are isolated via magnetic bead separation using the CliniMACS platform, and were then transduced with the lentiviral vector encoding the NY-ESO-1 TCR and sr39TK reporter/suicide gene (enabling non-invasive visualization of the transgenic HSC progeny in vivo within the bone marrow niche, as well as serve as a safety ablation feature), and were then cryopreserved. Following final lot release criteria for the gene-modified PBSCs, patients were admitted following unmobilized leukapheresis to collect peripheral blood mononuclear cells (PBMCs), which were then transduced with a retroviral vector encoding the NY-ESO-1 TCR while the patient received conditioning chemotherapy with busulfan and fludarabine in parallel, in order to selectively myeloablate and lymphodeplete the patient, respectively. Gene-modified PBSCs and PBMCs were administered to the patient on day 0 and day +1, respectively, and patients subsequently received 7 days of IL-2 for transgenic T-cell expansion in vivo. Created in BioRender. Nowicki, T. (2025) https://BioRender.com/4bcin2w.
The study shows that genetically modified stem cells can serve as a renewable internal source of tumour-targeting immune cells. This approach could overcome one of the major hurdles of current T cell therapies: short-lived immune responses.
The team genetically modified patients’ hematopoietic stem cells (HSCs), the blood stem cells that normally give rise to all immune cells, by inserting a cancer-targeting receptor (specific to NY-ESO-1, a tumour antigen found in sarcomas and melanomas). After bone marrow transplantation, these modified stem cells engrafted in the patients and began producing functional T cells that seek out and destroy cancer.
Why NY-ESO-1 and Sarcomas?
- NY-ESO-1 is a “cancer-testis antigen” present in multiple tumour types but rare in healthy tissues, making it a safe and specific target.
- Sarcomas, particularly synovial sarcoma, express NY-ESO-1 in ~80% of cases and have poor outcomes after relapse, making them ideal for a proof-of-concept trial.
What the Trial Showed
- In this early-stage clinical trial:
- Engineered stem cells successfully engrafted in all patients.
- T cells targeting the tumour were generated and persisted for months.
- One patient showed tumour regression.
- Advanced imaging confirmed that stem cells homed to the bone marrow and created a renewable T cell supply.
This study proves a bold idea: that human stem cells can be reprogrammed to generate an ongoing supply of precision-guided immune cells against cancer. It’s not yet a cure, but it’s a paradigm shift toward more durable, personalised, and preventive immunotherapy.
Journal article: Nowicki., T.S., et al, Human cancer-targeted immunity via transgenic hematopoietic stem cell progeny, Nature Communications.
Summary by Stefan Botha










