A new study identifies “mitoxyperilysis,” a previously unknown cell death pathway that could be harnessed to treat cancer (Figure 1).
In many diseases, from infections to cancer, immune activation and nutrient scarcity occur at the same time. Yet these two stress signals have rarely been studied together. Now, researchers have uncovered what happens when they overlap: cells undergo a brand-new form of inflammatory cell death.
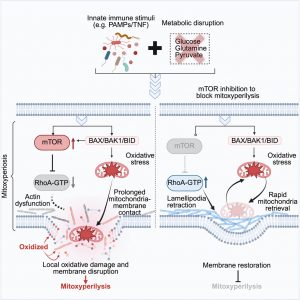
Published in Cell, the study describes this process as mitoxyperilysis, a mechanism in which damaged mitochondria cluster near the cell membrane, unleashing bursts of oxidative damage that cause the cell to rupture.
During infections or in growing tumours, the innate immune system is activated while local nutrients become scarce. The team combined these two conditions experimentally and observed something unexpected: mitochondria stopped roaming inside the cell and instead moved to the edge of the cell, remaining jammed against the membrane.
Damaged mitochondria generate reactive oxygen species (ROS), which can destroy cellular structures. Positioned at the membrane, these ROS effectively “burn through” the cell surface, leading to rupture and cell death.
The study revealed that the metabolic regulator mTOR is essential for this process. When mTOR was inhibited or genetically deleted, mitochondria stayed away from the membrane and cells survived.
Innate immune signalling was equally crucial: deleting a key innate immune receptor prevented mitoxyperilysis from occurring at all. Together, these findings show that mitoxyperilysis requires both metabolic stress and immune activation, making it a highly specific cellular response.
Because many cancers experience nutrient limitation and immune pressure, the researchers asked whether mitoxyperilysis could be therapeutically induced to kill tumour cells.
In mouse models, they paired:
- Fasting (to create metabolic stress), and
- Innate immune activation using a bacterial component
The result: within two days, tumours in fasted, immune-activated mice significantly regressed. Neither intervention alone reduced tumour size, but together they triggered widespread tumour cell death, with mitochondrial clustering and membrane rupture identical to mitoxyperilysis observed in earlier experiments.
The discovery highlights the potential of combination therapies that simultaneously manipulate metabolism and innate immunity. It also underscores the value of integrating concepts from typically siloed fields.
Scientists have identified a new form of cell death, mitoxyperilysis, triggered when immune activation and nutrient shortage occur together. This discovery not only fills a major gap in our understanding of cell biology but also points to innovative combination approaches for killing cancer cells.
Journal article: Wang, Y., et al. 2025. Innate immune and metabolic signals induce mitochondria-dependent membrane lysis via mitoxyperiosis. Cell.
Summary by Stefan Botha