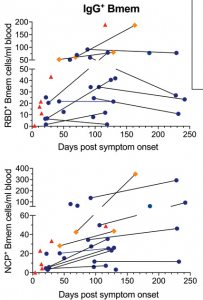
Absolute numbers of total IgG+ memory B cells specific for epitopes in the receptor binding domain (RBD+) nucleocapsid (NCP+) proteins. Samples are plotted by days post-symptom onset for 25 individuals, with 11 patients sampled twice and paired samples connected with gray lines. Patient datapoints are marked based on disease severity with severe as red triangles, moderate as orange diamonds and mild as blue circles. (Source: Hartley et al., 2020)
In just over year since SARS-CoV-2 emerged, new vaccines approved for emergency use have been developed and are currently being rolled out. Vaccination aims to induce long-lived immunity, however, a very important question is: how long does naturally induced SARS-CoV-2 immunity last? Multiple studies have demonstrated a significant decline in circulating SARS-CoV-2 specific antibodies as early as a month post-recovery, however, this only represents one aspect of adaptive immunity. Two recently published studies provide evidence for longer (-lived) SARS-CoV-2 immunity.
Hartley et al. aimed to determine the longevity and immunophenotype of SARS-CoV-2-specific memory B cells in COVID-19 patients. The immunophenotyped 36 samples (11 of which were paired) from individuals between 4 and 242 days post-COVID-19 symptom onset. They demonstrated that though serum levels of SARS-CoV-2-specific Abs decline and reach a plateau, SARS-CoV-2-specific memory B cells that express IgG1 (and IgM) persist long-term and continue to increase during convalescence. Based on their findings they propose “that vaccination studies could potentially use SARS-CoV-2 memory B cells as a surrogate marker of robust humoral immunity as circulating antibodies are known to wane rapidly following antigen clearance”.
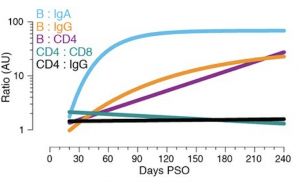
Relationships between immune memory compartments in COVID-19 subjects over time, as ratios (full curves and data shown in fig. S10, B to F). AU = arbitrary units, scaled from fig. S10, B to F. “B/IgA”, RBD-specific memory B cell ratio to Spike IgA antibodies. “B/IgG”, RBD-specific memory B cell ratio to RBD IgG antibodies. “B/CD4”, RBD-specific memory B cell ratio to SARS-CoV-2-specific CD4+ T cells. “CD4/CD8”, SARS-CoV-2-specific CD4+ T cells ratio to SARS-CoV-2-specific CD8+ T cells. “CD4/IgG”, SARS-CoV-2-specific CD4+ T cells ratio to RBD IgG antibodies. (Source: Dan et al., 2021)
Dan et al., performed a comprehensive analysis of multiple compartments of adaptive immunity: antibodies, B cells, CD8+ T cells, and CD4+ T cells using samples from 188 recovered COVID-19 patients. This large cohort included approximately 15% of samples taken more than 6-months post-infection. They also observed a significant increase in circulating memory B cells during convalescence. However, SARS-CoV-2-specific CD4+ T cells and CD8+ T cells declined with a half-life of 3-5 months. They conclude that despite observing subset specific differences in the durability of SARS-CoV-2 immunity, their data:
“show immune memory in at least three immunological compartments was measurable in ~95% of subjects 5 to 8 months post-symptom onset, indicating that durable immunity against secondary COVID-19 disease is a possibility in most individuals.”
In summary, both studies provide hope for long-lasting memory B cell immunity which persists despite a decline in circulating SARS-CoV-2-antibodies. Further, they highlight specific immune compartment that could be used as better markers of persisting immunity in vaccination studies.
Journal Articles:
- Hartley et al., 2020. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Science
- Dan et al., 2020. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science
Summary by Cheleka AM Mpande










