Researchers use a temporary “liver factory” to restore T cell strength in older mice (Figure 1).
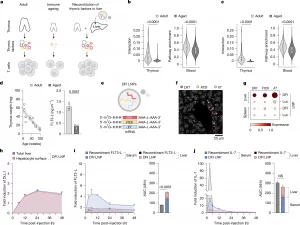
Figure 1: Hepatic reconstitution of declining T cell signalling factors to restore immune signalling in ageing. a, Overview of the approach to restore age-declining immune trophic cues by hepatic expression of Dll1, Flt3l and Il7 mRNAs. b, Spatial ligand–receptor interactions between thymic cortical epithelial cells (cTECs) and thymocytes decline with age (left), and ssGSEA shows reduced Notch pathway activity in circulating T cells (right). n = 47 spatial arrays and 96,683 blood T cell transcriptomes across 21 ages. Data are represented as violin plots with median + interquartile range. Statistical significance was determined by Mann–Whitney tests. c, cTEC–T cell IL-7 interaction (left) and downstream pathway in circulating T cell (right) activities are likewise diminished with age. n = 47 spatial arrays and 96,683 blood T cell transcriptomes across 21 ages. Data are represented as violin plots with median + interquartile range. Statistical significance was determined by Mann–Whitney tests. d, Thymus weight decreases with age (n = 18; 3 per timepoint). Interstitial FLT3-L levels are reduced in aged thymus by ELISA (n = 3 per group). Data are mean ± s.e.m.; statistical significance was determined by a two-tailed unpaired Student’s t-test. e, mRNA (DFI; Dll1, Flt3l and Il7) constructs formulated in SM-102 LNPs. f, Representative RIBOmap images 6 h post-DFI show robust ribosome-bound transcripts in the liver. A representative image from three imaged DFI-treated animals is shown. g, Single-cell quantification: translating Dll1, Flt3l and Il7 in the liver and spleen by RIBOmap (n = 1 for Luc and n = 3 for DFI). h, Immunofluorescence of DLL1 protein over 0–48 h after 5 µg DFI reveals transient induction in total liver and hepatocyte surface (phalloidin co-stain). Fold induction from baseline (0 h) is shown. n = 32 fields of view from n = 3 animals per time point per condition. Data are mean ± s.e.m. i, ELISA for FLT3-L levels in serum and the liver after 10 µg recombinant FLT3-L or 5 µg DFI at 3–48 h. Liver concentrations were normalized to liver weight; fold change from 0 h is shown. n = 3 animals per time point per compartment per condition. Data are mean ± s.e.m.; area under the cover (AUC) over 48 h compared by a two-tailed unpaired Student’s t-test. j, ELISA for IL-7 in serum and the liver after 10 µg recombinant IL-7 or 5 µg DFI at 3–48 h with liver normalization and fold change from 0 h. n = 3 animals per time point per compartment per condition. Data are mean ± s.e.m.; 48-h AUC compared by a two-tailed unpaired Student’s t-test. NS, not significant.
As we age, our immune system gradually loses its edge. The number and diversity of T cells decline, responses to vaccines weaken, and infections and cancers become harder to control. A major reason is the slow shutdown of the thymus, the organ responsible for producing and training new T cells, which becomes largely nonfunctional by old age.
Now, report a new approach to counteract this decline. The team show that temporarily reprogramming the liver with mRNA can rejuvenate T cell immunity in older mice, improving responses to both vaccines and cancer immunotherapy.
The thymus plays a critical role early in life by generating diverse T cells and releasing signals that help them survive and mature. But beginning in early adulthood, the thymus steadily shrinks in a process known as thymic involution. As thymic output falls, the immune system becomes increasingly reliant on aging, less adaptable T cells.
Rather than trying to regenerate the thymus itself, the researchers took a synthetic biology approach. They asked whether another organ could be induced to temporarily produce the same signals normally supplied by the thymus.
The liver was an ideal candidate. It remains highly active with age, is easy to target with mRNA therapies, and filters all circulating blood, including immune cells.
Using lipid nanoparticles, the team delivered mRNA encoding three critical immune-supporting factors directly to liver cells:
- DLL1, involved in T cell lineage commitment
- FLT-3, which supports immune progenitor survival
- IL-7, a key cytokine for T cell maintenance
Once inside liver cells, the mRNA instructed them to briefly produce these signals, effectively creating a temporary thymus-like environment in the bloodstream.
In experiments with 18-month-old mice (roughly equivalent to middle-aged humans), repeated mRNA dosing over four weeks led to:
- Larger and more diverse T cell populations
- Improved T cell function
When vaccinated, treated mice generated twice as many antigen-specific cytotoxic T cells as untreated mice of the same age. The approach also boosted cancer immunotherapy: mice receiving both the mRNA treatment and a PD-L1 checkpoint inhibitor survived significantly longer than those given immunotherapy alone.
Importantly, all three factors were required, no single signal could reproduce the immune rejuvenation on its own.
While the work is still preclinical, it highlights the potential of mRNA-based, reversible immune engineering, not to permanently alter tissues, but to provide timely immune support when natural systems decline.
By using mRNA to temporarily turn the liver into a source of T cell–supporting signals, researchers restored immune strength in aging mice. The approach improved vaccine responses and cancer immunotherapy, offering a promising new direction for promoting immune health during aging.
Journal article: Mirco J. Friedrich, M.J., et al. 2025.Transient hepatic reconstitution of trophic factors enhances aged immunity. Nature.
Summary by Stefan Botha










