Osteoarthritis (OA) is a prevalent and debilitating joint disease marked by the progressive degradation of cartilage, inflammation, and structural alterations. Typically diagnosed based on pain symptoms and joint stiffness, OA diagnosis currently relies on radiographic imaging, which often identifies joint damage only once it has significantly progressed. Recent advancements in biomarker research offer potential for earlier and more sensitive diagnosis, addressing the limitations of traditional radiographic methods.
Additional reading: From Gut to Joints: How Friendly Microbes May Trigger Autoimmunity in Rheumatoid Arthritis
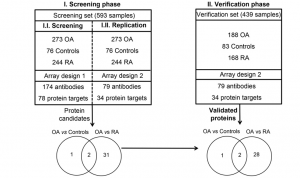
Schematic overview of the study. A screening phase was performed using a set of 593 serum samples and a protein array composed of 174 antibodies targeting 78 different proteins (Array 1, Phase I.I.). The levels of three proteins were found significantly (P < 0.05) different between osteoarthritis (OA) patients and control individuals, and 33 proteins differed between OA and rheumatoid arthritis (RA). A more focused array (Array 2) was then built targeting these 34 different proteins with 79 antibodies, and the results were replicated in the same sample set (Phase I.II.). Finally, a verification phase (II) was carried out using this second array on an independent set of 439 serum samples. In this phase, the three biomarker candidates separating OA and controls were verified, as well as 30 of the 33 proteins that were found with altered levels between OA and RA patients.
A recent study applied an affinity proteomic approach to screen serum samples from OA patients, healthy controls, and patients with rheumatoid arthritis (RA) to identify protein biomarkers that could aid in OA diagnosis. This investigation pinpointed three promising proteins—C3, ITIH1, and S100A6—that were consistently elevated in OA patients. These proteins play roles in inflammatory responses and cartilage stability, indicating their potential utility as early diagnostic markers. The study’s protein panel achieved an impressive classification accuracy (area under the curve > 0.82), distinguishing OA from healthy controls and from RA with notable precision.
These findings reveal insights into OA pathophysiology, as C3, ITIH1, and S100A6 are implicated in processes like complement activation, matrix stability, and cellular survival. In OA, complement proteins, such as C3, contribute to joint inflammation and cartilage degradation. ITIH1, a protein that binds to hyaluronic acid in cartilage, provides structural support, while S100A6, a calcium-binding protein, may influence cartilage cellular processes. This protein panel, therefore, represents a robust tool for OA detection and could significantly impact clinical practice, especially with early-stage interventions that slow disease progression.
Further validation, this biomarker-based method may offer a cost-effective and accessible way to diagnose and monitor OA. Such approaches hold promise for transforming OA management, enhancing patient quality of life, and enabling earlier therapeutic measures.
Summary by Faith Oluwamakinde










