Crohn’s disease and ulcerative colitis affect different regions of the digestive tract but share common features: painful abdominal cramps, diarrhoea, weight loss, and fatigue. These symptoms often arise in cycles, flaring unpredictably and placing significant stress on patients’ quality of life. In addition to tissue damage, long-term inflammation increases the risk of developing colorectal cancer.
While traditional therapies broadly suppress the immune system, newer treatments aim to interrupt specific pathways that drive inflammation. However, not all patients respond to these approaches, and the reasons for this variability have remained unclear—until now.
Chronic inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis, remain notoriously difficult to treat—especially for young people, in whom symptoms often emerge during critical years of education and career development. Now, researchers have identified a key driver of persistent gut inflammation, uncovering a promising new target for therapy (Figure 1).
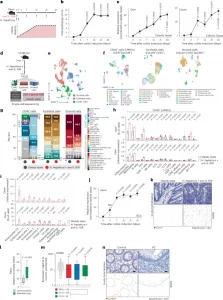
Figure 1: Epithelial cells upregulate OSMR in intestinal inflammation. a, Schematic of the H. hepaticus + anti-IL-10R-induced colitis time course study. b, Histopathology scores of colitis severity at days 3 (n = 6), 7 (n = 41), 14 (n = 23), 21 (n = 16) and >28 (n = 6), pooled from two to four experiments. Data are shown as mean ± s.e.m. and were analyzed by a Kruskal–Wallis test with a Dunn’s post hoc test. c, qPCR analysis of Osm and Osmr expression in colon tissue at days 3 (Osm/Osmr, n = 4/4), 7 (Osm/Osmr, n = 14/20), 14 (Osm/Osmr, n = 9/14), 21 (Osm/Osmr, n = 20/21) and >28 (Osm/Osmr, n = 10/13), normalized to steady-state controls. Data were pooled from two to four experiments and are shown as mean ± s.e.m. P values were calculated by (two-tailed) one-sample t-tests and Wilcoxon rank tests. d–i, Single-cell workflow: stromal (calcein⁺CD45⁻EpCAM⁻), immune (CD45⁺) and epithelial (EpCAM⁺) cells were sorted, pooled (three mice per pool, three pools per group) and processed with 10x Genomics (18 mice total). d, Experimental approach for single-cell workflow. e, Uniform manifold approximation and projection (UMAP) showing CD45⁺, epithelial and stromal cell clusters. f,g, UMAP (f) and quantification (g) of major cell populations within CD45+, epithelial and stromal clusters; DCs, dendritic cells; LPMCs, lamina propria mononuclear cells; NK, natural killer; Treg, regulatory T cells; TA cells, transit-amplifying cells. h, Relative Osm expression and percentage of Osm⁺ cells within the CD45⁺ cluster. i, Relative Osmr expression and Osmr⁺ cell frequency within epithelial clusters. Data are shown as mean ± s.e.m. and were analyzed by Wilcoxon rank-sum test; NS, not significant. j, qPCR analysis of epithelial Osmr expression at days 3, 7, 14, 21 and >28 (n = 4, 23, 12, 15 and 11, respectively), normalized to steady-state controls and pooled from two to four experiments. Data are shown as mean ± s.e.m. P values were calculated by (two-tailed) one-sample t-tests and Wilcoxon tests. k, ISH of Osmr in colon tissue from colitic (day 10, n = 3) and steady-state mice (n = 6). Purple signal indicates Osmr expression. Data were analyzed by ilastik-based quantification; scale bar, 20 μm. l, Quantification of Osmr⁺ cells by ISH in the lamina propria (pink) and epithelium (green) of colitic (day 7) versus steady-state mice (n = 6). Data were analyzed by Mann–Whitney U-test. m, qPCR of epithelial OSMR expression in healthy individuals and individuals with IBD (UC, n = 10; CD, n = 8). Data are shown as mean ± s.e.m.; statistical tests used were the same as in j. n, ISH of OSMR in mucosal biopsies from healthy individuals (n = 5) and individuals with UC (n = 12); brown dots indicate OSMR⁺ cells. Dashed lines demarcate the epithelium; scale bar, 20 μm.
The team has uncovered a harmful interaction between two immune signalling molecules—oncostatin M (OSM) and interleukin-22 (IL-22)—that fuels chronic gut inflammation.
IL-22 typically plays a protective role in maintaining the gut’s epithelial barrier, but in the inflamed intestine, it appears to inadvertently heighten sensitivity to OSM by increasing the number of its cellular receptors. OSM, in turn, acts like an inflammatory amplifier. Produced by immune cells, it triggers a cascade of signals that intensify the immune response, drawing even more inflammatory cells into the gut lining—ultimately sustaining the chronic inflammation that characterizes IBD.
The study used single-cell sequencing and tissue analysis from both animal models and human patients. It revealed that inflamed intestinal tissues—especially near tumour sites—had far more OSM receptors than healthy areas. This finding not only links OSM to disease severity but also suggests a role in promoting inflammation-associated colorectal cancer.
Importantly, high OSM levels were correlated with treatment failure in some patients, highlighting the molecule’s potential as both a therapeutic target and a biomarker to predict who may not respond to current therapies.
The team’s findings have already progressed toward clinical application. As research continues, this work marks a promising advance in unraveling the complexities of IBD—and in designing smarter, more specific ways to restore balance to the immune system.
Journal article: Cineus, R., et al., 2025. The IL-22–oncostatin M axis promotes intestinal inflammation and tumorigenesis. Nature Immunology.
Summary by Stefan Botha










