Lung transplantation offers a vital lifeline for patients with end-stage lung disease, yet more than 50% of lung-transplant recipients experience organ rejection within five years. Despite decades of research, the biological mechanisms driving this chronic rejection have remained elusive.
Recently, scientists have provided the most detailed picture yet of how lung damage and transplant rejection unfold at the cellular level (Figure 1). Their study reveals that abnormal cross-talk between donor and recipient cells drives progressive scarring, leading to chronic rejection and organ failure.
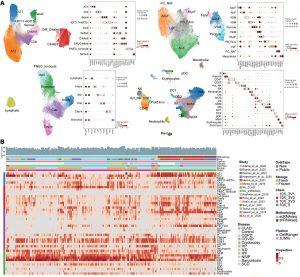
Figure 1: Integrated object of single-cell transcriptome from multiple datasets. (A) Uniform manifold approximation and projection (UMAP) plot displaying cell types, accompanied by a dot plot showing the expression levels of key marker genes. The expression level was z-score–transformed. AMs, alveolar macrophages; IM, interstitial macrophages; MoM, monocyte-derived macrophages. (B) Heatmap of cell type proportions across samples, where each row represents a cell type, and each column represents a sample. Diff_Ciliated, differentiating ciliated; PNEC_Ionocyte, pulmonary neuroendocrine cells and ionocyte; ASM, airway smooth muscle; VSM, vascular smooth muscle; FibM, fibromyocyte; LipF, lipofibroblast; PC_NAF, perichondrial or nerve-associated fibroblast; Cap, general capillary endothelial cells; Cap-a, capillary aerocyte; NCM, nonclassical monocytes; Act_NK, activated NK; pDC, plasmacytoid dendritic cells; Prolif.Imm, proliferative immune cells.
Surgeons perform approximately 3,000 to 3,500 lung transplants annually in the United States, but chronic lung allograft dysfunction (CLAD), an umbrella term for chronic rejection syndromes, remains the leading cause of death beyond the first year post-transplant. Once CLAD develops, there are currently no effective treatments beyond repeat transplantation.
To uncover the mechanisms behind this condition, the Northwestern team analyzed 1.6 million single cells from transplanted lungs. By distinguishing between donor-derived structural cells and recipient immune cells, the researchers discovered that these two cell types “talk” to one another through harmful signaling loops that promote inflammation and scarring.
Among the most striking findings was the identification of rogue structural cell types, marked by KRT17 and KRT5 expression, that actively drive fibrosis across multiple lung diseases: including idiopathic pulmonary fibrosis (IPF), interstitial lung disease (ILD), COPD, COVID-19–associated lung damage, and transplant rejection.
By integrating data from multiple scarring lung diseases, the team generated a comprehensive reference map outlining both shared and disease-specific molecular pathways. This integrated analysis not only explains why rejection occurs but also offers clues for cross-disease therapeutic strategies.
The study also identified previously unrecognized immune cell populations involved in chronic rejection:
- “Exhausted” T cells that remain chronically activated but lose their ability to regulate inflammation.
- “Super-activated” macrophages that persistently promote tissue damage and fibrosis.
Together, these immune cells create a feed-forward inflammatory network, sustaining scarring long after the initial immune response has subsided.
Advanced computational methods allowed the team to integrate diverse datasets from multiple studies, overcoming previous technical barriers and enabling a unified analysis of immune and structural interactions across disease types.
The researchers pinpointed several key molecular drivers of fibrosis, including PDGF, GDF15, and TWEAK signaling pathways, opening new avenues for drug discovery.
Notably, existing antifibrotic medications such as nintedanib (Ofev) and pirfenidone (Esbriet), already approved for pulmonary fibrosis, may be repurposed to prevent transplant rejection, offering immediate translational potential.
While the research was focused on chronic transplant rejection, its implications reach far wider. The molecular pathways and cell types identified are central to many forms of pulmonary fibrosis, affecting hundreds of thousands of patients worldwide.
Journal article: Yan., Y., et al. 2025. Single-cell dissection of chronic lung allograft dysfunction reveals convergent and distinct fibrotic mechanisms, JCI Insight.
Summary by Stefan Botha










