The prevalence of hay fever has been steadily rising, fuelled by factors such as climate change, pollution, urban lifestyles, and altered immune development due to modern hygiene practices.
But now, a proof-of-principle study offers a new ray of hope. Researchers have engineered a monoclonal antibody that, when applied directly to the inside of the nose, protected mice from developing allergy and asthma-like symptoms caused by mugwort pollen (Figure 1).
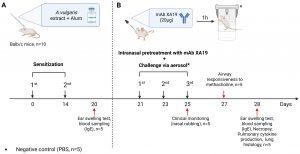
Figure 1. Study design. The schematic illustration shows the sensitization of mice with Artemisia vulgaris pollen extract (A), intranasal pretreatment with the monoclonal antibody XA19 and following subsequent allergen challenge (B).
This marks the first time a monoclonal antibody targeting a specific pollen allergen has been delivered nasally and shown to prevent both upper and lower airway symptoms.
Traditional allergen immunotherapy gradually desensitizes patients by exposing them to controlled doses of the allergen. While effective for some, it’s a slow process, and not all patients respond. More recently, allergen-specific monoclonal antibodies have emerged as an alternative. These antibodies typically work by:
- Blocking allergens directly to prevent them from triggering an immune reaction
- Neutralising IgE antibodies, which drive allergic responses
However, existing monoclonal antibody therapies are injected into the bloodstream, requiring clinic visits and systemic distribution.
The team first immunised mice with mugwort pollen, prompting them to produce anti-pollen antibodies. After harvesting the mice’s spleen cells, the researchers fused them with lab-grown myeloma (cancer) cells to create five immortal “hybridoma” cell lines, each producing a single antibody type. Rigorous testing identified hybridoma cell line XA19 as producing the most potent antibody, which was selected for further development.
To assess efficacy, the XA19 antibody was applied inside the noses of mice that had been made allergic to mugwort pollen. Control mice were either sensitized but given a placebo or left unsensitised altogether.
After repeated nasal exposures to mugwort pollen:
- Treated mice showed significantly fewer allergy symptoms, including reduced ear swelling (a marker of allergic inflammation in rodents).
- They rubbed their noses less frequently, indicating less nasal irritation.
- Their lung function remained intact, while untreated allergic mice experienced airway restriction.
- Inflammatory molecules (cytokines) in the lungs were substantially lower.
In short, the nasal antibody treatment blocked the allergic cascade, preventing the pollen from triggering IgE-mediated reactions in the respiratory system.
As rising pollen counts driven by climate change worsen the allergy burden worldwide, such innovative precision therapies could significantly improve quality of life for millions once validated and successfully trialed in humans.
Journal article: Tabynov, K. et al., 2025. Intranasal monoclonal antibodies to mugwort pollen reduce allergic inflammation in a mouse model of allergic rhinitis and asthma. Frontiers in Immunology.
Summary by Stefan Botha










