Despite the huge progress being made in recent years, multiple myeloma (MM) remains a challenging disease where the clinical laboratory plays a pivotal role in the management of patients. This disease is a complex and heterogeneous hematological malignancy characterized by the proliferation of clonal plasma cells in the bone marrow. The substitution of normal plasma cells with multiple myeloma cells and the overproduction of a monoclonal protein (MP) lead to the development of organ damage summarized in the acronimun CRAB: hypercalcemia, renal insufficiency, anemia and bone lesions.
In this review, Rios-Tamayo, et al., summarize the current basic laboratory testing in MM patients and propose a new algorithm to manage and follow up the MP based on the heavy/light chain assay (HLC, Hevylite®; The Binding Site).
Currently, monoclonal intact immunoglobulins (Igs) and free light chains (FLC) secreted by malignant plasma cells are considered as biomarkers of tumor burden, in fact, in the international guidelines recommend the detection and quantification of these MPs by using complementary techniques including serum and urine protein electrophoresis (SPEP/UPEP), serum and urine immunofixation (sIFE/uIFE) and the serum FLC assay (Freelite; The Binding Site). Even though, analyzing the MP is crucial in the daily clinical practice, the conventional techniques (PEP and IFE) are associated with some issues such as heterogeneous MP migration (for example, IgA), low analytical accuracy for low MP concentration and interlaboratory variability, so new more sensitive, specific and reliable test could help to better determine the MP.
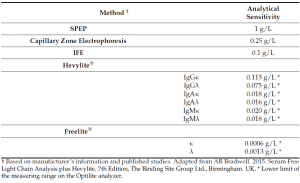
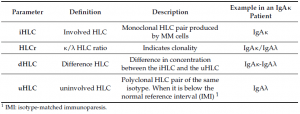
Hevylite® is a fully automatized immunoassay that specifically detects the different heavy/light chain (HLC) pairs (IgGκ/IgGλ, IgAκ/IgAλ, IgMκ/IgMλ), thus providing an accurate quantification of the involved/monoclonal HLC (iHLC) pair and the uninvolved/polyclonal HLC (uHLC) pair of the same isotype. Importantly, the limit of detection of the HLC assay is lower than conventional methods (SPEP, Capillary Zone Electrophoresis and IFE) currently used for the detection of monoclonal intact Igs in serum.
Numerous studies have confirmed the good correlation between the summation of the iHLC and the uHLC concentrations with total immunoglobulin IgG, IgA and IgM levels, demonstrating the accuracy of the assay to quantify mono and polyclonal Igs. Similar to the serum FLC test, the HLC assay provide a sensitive measurement of clonality through the HLC ratio (rHLC) with a global diagnostic sensitivity closet o 99%.
Regarding its prognostic value, in large cohorts of newly diagnosed MM patients, an abnormal rHLC is associated with shorter overall survival. Moreover, the degree of alteration of the rHLC has been related to the response achieved in treated MM patients, where a lower iHLC/uHLC ratio correlates with a deeper response reached.
Based on the information the could be achieved by the Hevylite assay, the proposed serological algorithm for the management of MP in patients with MM is:
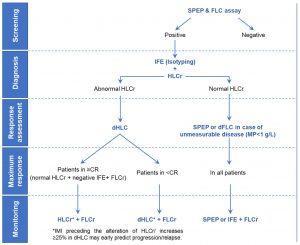
Figure 1: Proposed serological algorithm for the management of the monoclonal protein in patients with multiple myeloma.
Journal article: Rios-Tamayo, et al. 2021. The Current Role of the Heavy/Light Chain Assay in the Diagnosis, Prognosis and Monitoring of Multiple Myeloma: An Evidence-Based Approach. Diagnostics.
Summary by Dr. Christian Barreto-Vargas