Researchers have identified a bioactive compound that may offer relief for people suffering from conditions marked by excessive immune responses—including irritable bowel syndrome (IBS), chronic itching, asthma, and migraine (Figure 1). The the study presents a receptor-blocking molecule that could prevent dangerous inflammatory reactions and potentially life-threatening outcomes such as anaphylactic shock.
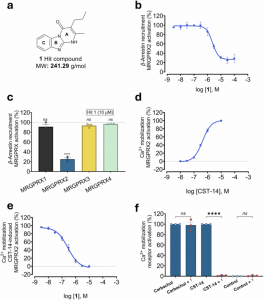
Figure 1: Characterization of MRGPRX2 inhibitor 1. a Chemical structure and molecular weight (MW) of 1. b Concentration-response curve of 1 determined in β-arrestin recruitment assays. CHO cells that recombinantly express MRGPRX2 were employed versus the agonist CST-14 (at its EC80 value of 1.0 µM). An IC50 value of 2,420 ± 220 nM was determined for 1. Data are means ± SEM of 3 biological replicates, performed in duplicates. c MRGPRX subtype selectivity of 1 (10 µM) determined in β-arrestin assays using CHO cells that recombinantly express the respective MRGPRX subtype. MRGPRX1 was activated by BAM-22P (8.5 µM),19 MRGPRX2 was activated by CST-14 (1 µM), MRGPRX3 was activated by PSB-20294 (1 µM), and MRGPRX4 by PSB-18061 (2.6 µM).44 Data are means ± SEM of 3 biological replicates, performed in duplicates. The effect of each MRGPRX agonist was assessed in the absence and in the presence of 1; results were normalized to the maximal agonist effect. Statistical significance was determined by one-way ANOVA (Tukey’s multiple comparisons) tests (ns, P > 0.05; ****P < 0.0001). d CST-14 Concentration-dependent activation of MRGPRX2 in LN229 cells overexpressing MRGPRX2, showing an EC50 value of 452 ± 49 nM. Data are means ± SEM of 3 biological replicates, performed in duplicates. e Concentration-inhibition curve of 1 determined in Ca2+ assays in LN229 cells overexpressing MRGPRX2 versus the agonist CST-14 (at its EC80 value of 800 nM), resulting in an IC50 value of 683 ± 174 nM. Data are means ± SEM of 3 biological replicates, performed in duplicates. f Compound 1 (10 µM) was tested versus the muscarinic agonist carbachol (100 µM) in the LN229-MRGPRX2 cell line, which endogenously expresses muscarinic acetylcholine receptors (mAChRs). Data are means ± SEM of 3 biological replicates. Statistical significance was determined by one-way ANOVA (Tukey’s multiple comparisons) test (ns P > 0.05 and ****P < 0.0001)
The breakthrough centres around the MRGPRX2 receptor, found on mast cells—immune cells loaded with inflammatory messengers that are typically activated during allergic responses. While mast cells are commonly triggered by antibody-antigen interactions (like in mosquito bites), they can also be activated without antibodies, leading to hard-to-treat allergic and inflammatory conditions.
MRGPRX2 was identified over a decade ago, but until now, scientists lacked tools to selectively block it and calm the immune response without disrupting other processes.
Key findings:
- A single compound was discovered to bind and block MRGPRX2
- Chemical modifications improved its potency and effectiveness at extremely low doses
- In mice, the compound prevented life-threatening allergic reactions
- In human mast cells, the compound also prevented release of inflammatory messengers
The molecule has since been refined to last longer in the body and selectively block only MRGPRX2—lowering the risk of side effects.
The therapeutic potential is far-reaching:
- Asthma and IBS – chronic inflammatory conditions driven by immune overactivation
- Chronic itch and allergic skin diseases – difficult to treat and severely impact quality of life
- Anaphylactic shock prevention – especially after certain drug administrations
- Neuroimmune disorders and migraines – where mast cell dysregulation is implicated
By calming mast cell activation at the source, the new compound could reshape treatment for millions of patients with immune-triggered conditions. Blocking MRGPRX2 might offer the precision immunomodulation medicine has long sought.
Journal article: Ghazl Al Hamwi. G., et al., 2025. Subnanomolar MAS-related G protein-coupled receptor-X2/B2 antagonists with efficacy in human mast cells and disease models. Signal Transduction and Targeted Therapy.
Summary by Stefan Botha










