The battle against malaria has led to various strategies, with malaria transmission-blocking vaccines (TBVs) emerging as a promising approach. TBVs do not directly protect the vaccinated individual but work by preventing the malaria parasite’s development within the mosquito host, thereby reducing transmission to humans. Traditional TBVs have typically relied on protein-protein conjugate formulations, which, while effective, sometimes lack the durability of immune response required for high-impact prevention. Recent advances in mRNA vaccine technology, especially demonstrated through COVID-19 vaccines, have brought renewed interest in this platform for infectious diseases, including malaria.
Extra reading: Targeting a Mosquito’s Weak Spot: A New Strategy for Malaria Control
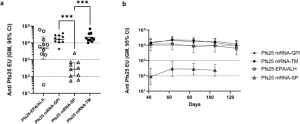
Anti-Pfs25 antibody levels in sera from mice vaccinated with Pfs25 mRNAs consisting of SP alone or SP with GPI anchor or TM, compared to Pfs25-EPA conjugate in Alhydrogel®. The figure shows the antibody levels on day 84 following vaccinations on days 0 and 21. b Serum antibody levels of all four immunogens at various time points from day 42 to day 126. Error bars represent the 95% confidence limit of the geometric mean. Statistical differences between groups were measured using a Kruskal–Wallis one-way ANOVA followed by a Dunn multiple comparator test. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001,
In a study investigating mRNA vaccines targeting malaria, researchers evaluated two leading antigens, Pfs25 and Pfs230D1, which play crucial roles in blocking transmission. These antigens were engineered into mRNA constructs with signal peptides (SP), glycosylphosphatidylinositol (GPI) anchors, and transmembrane (TM) domains to optimize their presentation on cell surfaces. The study found that mRNA vaccines incorporating GPI or TM domains displayed strong antigen expression on cell surfaces, which, when administered in mouse models, led to durable immune responses. Vaccines with GPI or TM domains showed transmission-reducing activities over 99% that persisted for over four months.
The immune responses triggered by these mRNA vaccines proved to be more robust and persistent than traditional protein-protein conjugates in comparative studies. Notably, antibody titres remained elevated without significant decline, underscoring the potential of mRNA platforms for sustained immunogenicity. This durability suggests that mRNA vaccines might overcome some limitations associated with protein-based malaria vaccines, potentially leading to longer-lasting community protection in high-risk areas. While promising, these findings also point to the need for further studies in non-human primates and human trials to confirm efficacy and longevity.
This research highlights a critical advance in malaria vaccine development, offering hope that mRNA vaccines could play an integral role in future malaria eradication efforts by interrupting transmission cycles at the mosquito level.
Summary by Faith Oluwamakinde










