Iron is indispensable for nearly all forms of life, and depriving pathogens of it is a central tactic in the body’s fight against infection. Immune cells, such as macrophages, often respond to bacterial invasion by pumping iron out of intracellular compartments, using transporters like NRAMP1, to limit bacterial growth. This usually slows or halts infection.
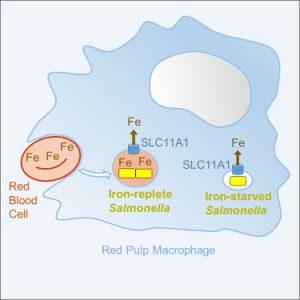
The human immune system employs a clever strategy to combat bacterial invaders: it starves them of iron, an essential nutrient for survival and replication. Yet some pathogens, like Salmonella, have evolved sophisticated ways to sidestep this defence. New research reveals that Salmonella bacteria actively seek out and exploit iron-rich niches within immune cells to continue proliferating—even under immune pressure (Figure 1).
This study has uncovered how Salmonella subverts this system. Using single-cell analysis in mouse models, the researchers found that a subset of Salmonella cells preferentially infects macrophages that naturally harbor high levels of iron. These cells are commonly found in the spleen, where old red blood cells—rich in iron—are broken down. The bacterium exploits these iron-abundant environments, using residual nutrients to sustain its replication.
Interestingly, even though NRAMP1 continues to extract iron from these intracellular compartments, small amounts remain—enough to support Salmonella’s survival and proliferation. This dual behaviour results in two distinct bacterial populations: one struggling in iron-depleted environments, and another thriving in iron-rich vesicles derived from red blood cell degradation.
The findings emphasize how bacterial pathogens can adapt to even well-established immune defence mechanisms. By targeting specific cellular niches, Salmonella effectively evades nutrient deprivation and drives ongoing infection.
Journal article: Roche, B., et al. 2025. A Salmonella subset exploits erythrophagocytosis to subvert SLC11A1-imposed iron deprivation. Cell Host & Microbe.
Summary by Stefan Botha