New research reveals an unexpected immune-driven cause of chemotherapy-induced nerve damage and a possible way to prevent it (Figure 1).
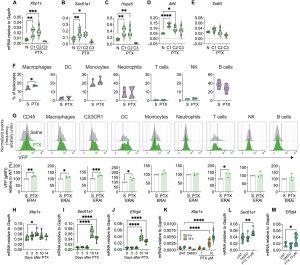
Figure 1: Paclitaxel activates IRE1α in immune cells in the peripheral nociceptive neuroaxis. (A to E) PBMCs from WT C57BL/6J mice (n = 6 or 7) were collected after paclitaxel (PTX) treatment on day 0 [cycle 1 (C1)], day 2 (C2), and day 4 (C3) or from naïve mice (N), and mRNA was measured by quantitative RT-PCR. (A) Xbp1s, (B) Sec61a1, (C) Hspa5, (D) Atf4, and (E) Ddit3. (F) Flow cytometry analysis of CD45+ immune cell subsets infiltrating the DRGs from ERAI mice treated with three doses of PTX (2 mg/kg, ip, on alternate days) or saline (S). n = 4; each n represents an independent cohort pooling four mice. (G) Representative histograms (gray, saline; green, PTX) and quantification of geometric mean fluorescence intensity (gMFI) for VFP expression in DRGs obtained by flow cytometry. Data are normalized to each cohort’s saline control (ERAI saline), which was given a value = 1. n = 4; each n represents an independent cohort pooling four mice. (H to J) DRGs from WT mice (n = 6 or 7) treated with PTX (2 mg/kg, ip, on alternate days) were collected at 0, 3, 5, 10, and 14 days posttreatment for RT-PCR quantification. (H) Xbp1s, (I) Sec61a1, and (J) ERdj4. (K) BMDMs (n = 6 to 8 replicates) were left untreated (UNT), vehicle-treated [dimethyl sulfoxide (DMSO)], or exposed to PTX (1 or 10 μM) for 6 or 18 hours, and Xbp1s expression was measured by RT-PCR. (L and M) BMDMs (n = 10 to 16 replicates) were treated as in (K), and expression of the indicated transcripts was assessed by quantitative RT-PCR, normalized to Gapdh. (L) Sec61a1 and (M) ERdj4. [(A) to (F) and (H) to (M)] Violin plots show median ± quartiles. (G) Data shown as means ± SEM. Statistical analysis: one-way ANOVA (Tukey’s test) for (A) to (E), (H) to (J), (L), and (M); two-tailed Student’s t test for (F) and (G); two-way ANOVA (Tukey’s test) for (K). *P < 0.05; **P < 0.005; ***P < 0.0005; ****P < 0.0001.
Chemotherapy has long been a double-edged sword: while it kills cancer cells, it can also leave patients with debilitating nerve pain. Up to half of all people receiving chemotherapy experience chemotherapy-induced peripheral neuropathy (CIPN) – tingling, numbness, or burning pain in the hands and feet, often so severe that treatment must be stopped early.
Researchers have identified a surprising culprit: not the nerves themselves, but immune cells responding to stress. Their findings uncover a molecular chain reaction that links cellular stress, inflammation, and nerve damage and point to a promising strategy to protect patients.
The team focused on a cellular stress pathway called IRE1α-XBP1, a kind of molecular “alarm system” that is switched on when cells are under duress. Using mouse models of chemotherapy, they found that the common cancer drug paclitaxel triggers immune cells to produce excessive reactive oxygen species, molecules that cause internal stress. This stress activates the IRE1α switch, pushing immune cells into a hyper-inflammatory state.
These overactive immune cells then migrate to the dorsal root ganglia, clusters of sensory neurons connecting the limbs to the spinal cord, where they release inflammatory molecules that irritate and damage nerve fibers, creating the hallmark symptoms of CIPN: pain, cold sensitivity, and nerve loss.
When the researchers silenced IRE1α in immune cells using genetic tools, they prevented this inflammatory surge and significantly reduced pain behaviours in mice. Even more promising, when they combined paclitaxel with a drug that inhibits IRE1α the mice showed fewer signs of nerve damage and maintained healthier nerves.
Because IRE1α inhibitors are already being tested in patients with advanced solid tumours (where the same pathway drives therapy resistance), the discovery opens the door to a dual-benefit therapy, drugs that both enhance cancer treatment and protect against nerve damage.
In a small pilot study, the team also analysed blood samples from women undergoing paclitaxel treatment for gynaecologic cancers. They found that patients who went on to develop severe neuropathy had higher IRE1α-XBP1 activation in their immune cells even before symptoms appeared, suggesting a potential biomarker for identifying high-risk patients.
Such a blood test could allow doctors to personalize therapy using preventive measures, such as IRE1α inhibitors, before irreversible nerve damage occurs.
This study reframes chemotherapy-induced nerve pain as an immune-driven inflammatory condition, not just nerve toxicity. By targeting the stress-sensing IRE1α pathway, researchers may soon be able to protect patients’ nerves while keeping cancer therapy on track.
Journal article: Fonseca, M. M., et al. 2025. Leukocyte-intrinsic ER stress responses contribute to chemotherapy-induced peripheral neuropathy. Science Translational Medicine.
Summary by Stefan Botha










