New strategies aim to overcome the biggest barrier to CAR-T success beyond blood cancers (Figure 1).
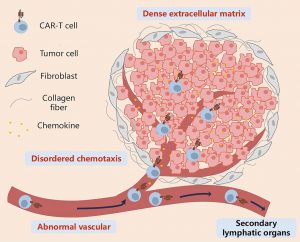
Figure 1: Key features that prevent CAR-T cells from infiltrating solid tumors through the circulation. CAR-T cells migrate to tumor tissues through the bloodstream under the guidance of chemokines, cross the abnormal tumor vasculature, penetrate the vicinity of tumor cells by overcoming barriers posed by the extracellular matrix and stromal cells, and ultimately generate stable intercellular contacts with tumor cells. During this process, disordered chemotaxis, abnormal tumor neovascularization, mesenchymal stromal cells dominated by cancer-associated fibroblasts, and a dense extracellular matrix are the main barriers that prevent CAR-T cells from effectively contacting tumor cells. CAR-T: Chimeric antigen receptor T.
Chimeric antigen receptor T-cell (CAR-T) therapy has transformed the treatment of blood cancers, delivering remarkable remission rates in leukaemia and lymphoma. Yet in solid tumours, CAR-T cells have struggled to deliver the same impact. The reason is not a lack of killing power, it’s access.
A new review outlines how researchers are tackling the central challenge: getting CAR-T cells into the solid tumor microenvironment (TME) and keeping them functional once they arrive.
Unlike blood cancers, solid tumours are protected by multiple physical and biological barriers:
- Abnormal blood vessels that limit immune cell trafficking
- A dense extracellular matrix (ECM) that acts like a physical wall
- Disrupted chemokine signals that fail to guide T cells to tumours
- Immunosuppressive stromal cells, including cancer-associated fibroblasts
As a result, circulating CAR-T cell levels in solid tumour patients are 5–10 times lower than in patients with hematologic cancers.
One promising approach is vascular normalisation. Anti-angiogenic drugs such as bevacizumab (anti-VEGF) can remodel chaotic tumour blood vessels, improving oxygenation and immune cell entry. When combined with CAR-T therapy, these agents enhance T-cell infiltration and antitumor effects in preclinical models. Other strategies target endothelial signalling and metabolism.
Another breakthrough involves rewiring chemokine signalling. Researchers are genetically engineering CAR-T cells to:
- Secrete chemokines such as CCL19 or CXCL10, or
- Express matching chemokine receptors like CXCR6
These modifications create directional signals that actively draw CAR-T cells into tumours. Early clinical evidence supports this approach: a phase I trial in hepatocellular carcinoma using glypican-3 CAR-T cells co-expressing CCL19 and IL-7 showed encouraging infiltration and activity.
The review also highlights methods to dismantle the tumour’s physical defences:
- Targeting fibroblast activation protein (FAP) on cancer-associated fibroblasts
- Using enzymes such as hyaluronidase to degrade ECM components
- Employing synNotch CAR-T cells that release matrix-degrading enzymes only within the tumour
These approaches have significantly improved CAR-T penetration and tumour control in preclinical studies.
CAR-T efficacy improves further when combined with other treatments:
- Chemotherapy (e.g., nab-paclitaxel) to loosen stromal structure
- Radiotherapy to induce inflammatory signals
- Oncolytic viruses to reshape the tumour microenvironment
Innovative local delivery methods including intratumoral injection, biomaterial scaffolds, and oxygen-releasing systems, can also increase CAR-T concentration at the tumour site while limiting systemic toxicity.
What Still Needs Solving
Despite rapid progress, major challenges remain:
- Translating complex preclinical models into human success
- Optimizing CAR-T cell phenotypes for hostile solid-tumour environments
- Scaling biomaterial-based delivery strategies for clinical use
The authors emphasize that future breakthroughs will depend on integrating immunology, genetic engineering, and materials science to design personalized, tumour-specific CAR-T therapies.
CAR-T cells can kill solid tumours if they can reach them. By remodelling blood vessels, guiding T-cell migration, breaking down physical barriers, and using smart combination therapies, researchers are steadily turning solid tumours into accessible targets for next-generation CAR-T immunotherapy.
Journal article: Wang, S., et al. 2025. Strategies and challenges in promoting chimeric antigen receptor T cells trafficking and infiltration of solid tumors. Chinese Medical Journal.
Summary by Stefan Botha










