A new gene therapy has successfully restored long-term immune function in 59 of 62 children born with ADA-SCID (adenosine deaminase deficiency–severe combined immunodeficiency), a rare and fatal genetic disorder that leaves children without a functioning immune system (Figure 1).
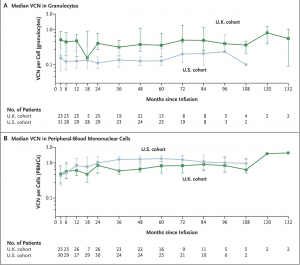
Figure 1: Median Vector Gene Marking in Granulocytes and Peripheral-Blood Mononuclear Cells. Shown is the median vector copy number (VCN) in granulocytes (Panel A) and peripheral-blood mononuclear cells (Panel B) from the first follow-up after infusion of the drug product (month 3) to the latest follow-up. 𝙸 bars indicate standard errors.
The study represents the largest and longest follow-up of any gene therapy for an inherited immune disease, marking a major milestone in regenerative medicine and clinical immunology.
ADA-SCID is caused by mutations in the ADA gene, which encodes an enzyme essential for the survival and development of immune cells. Without it, children are born unable to fight infections, a condition once known as “bubble baby syndrome.” Without treatment, most affected infants die before their second birthday.
Current options, bone marrow transplantation or enzyme replacement therapy, are lifesaving but limited by donor availability, incomplete immune recovery, and long-term side effects.
The new gene therapy offers a transformative alternative. Researchers collect a patient’s own blood stem cells, introduce a healthy copy of the ADA gene using a lentiviral vector, and reinfuse the corrected cells. Over time, these gene-modified stem cells produce healthy immune cells capable of fighting infections.
With its long-term success, safety, and scalability, this ADA-SCID gene therapy sets a precedent for treating other inherited immune and blood disorders, including sickle cell disease, Wiskott-Aldrich syndrome, and chronic granulomatous disease.
By demonstrating that one-time genetic correction can deliver lasting immune protection, this work underscores the promise of curative immunogenetics, where a patient’s own cells become the therapy.
Journal article: Booth, C., et al. 2025. Long-Term Safety and Efficacy of Gene Therapy for Adenosine Deaminase Deficiency. New England Journal of Medicine.
Summary by Stefan Botha










