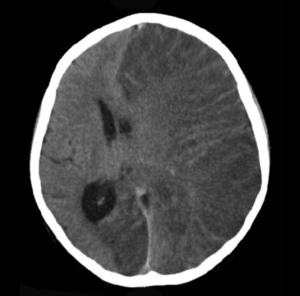
Brain CT scan without contrast enhancement of a patient, female, 8 years old, with Rasmusen’s encephalitis. There is severe swelling of the left cerebral hemisphere with shift of the midline structures. The patient is comatose with epilepsia partialis continua. Mikhail Kalinin -Own Work (Wikimedia Commons)
Rasmussen’s encephalitis (RE) is a rare inflammatory neurological disease characterised by frequent seizures. The only successful treatment available for RE is functionally disconnecting or completely removing the affected part of the brain. A major challenge in developing therapies against RE is limited knowledge about its origin and pathology of the disease. Furthermore, no suitable experimental model of RE disease existed. Thus, Kebir et al., aimed to develop an experimental murine model of RE in that recapitulates aspects of human pathology and can be used to evaluate candidate RE therapeutics.
Central nervous system (CNS)- targeted inflammation characterised by increased T cell infiltration is the most consistent immunopathology associated with RE. Based on this Kebir et al., hypothesised that injecting NSG mice with peripheral blood mononuclear cells (PBMCs) from RE patients would induce RE. Indeed researchers observed that injecting mice with cells and not plasma from RE patients resulted in development of seizures in 84% of NSG mice engrafted with PBMC from RE patients. Using flow cytometry analysis of brain samples from mice, researchers demonstrated that IFN-γ, IL-17 and granzyme-B expressing CD4 T cells and HLA-DR+ lymphocytes contribute to CNS inflammation and development of seizures.
Finally, they used the murine RE model to determine the prophylactic and therapeutic effects of anti-α4-intergrin blocking antibody against RE. In both cases anti-α4-intergrin blocking antibody treatment resulted in reduced levels of lymphocyte infiltrations, as well as reduced levels of IFN-γ and IL-17-producing T cells in the CNS. However, prophylatic and not therapuetic RE treatment prevented seizure development. Highlighting that reducing lymphocyte infiltration using a therapeutic in not sufficient to prevent seizures.
In summary, this research represents the first an animal model of RE. RE is a very rare disease that is challenging to diagnose, this model has the potential to be used as a confirmatory diagnostic, as well as evaluate therapeutic efficacy.
*NSG mice: NOD/Ltsx-scid IL-2Rgc (null) mice lack mature T and B cells, functional NK cells and are deficient in cytokine signaling.
Article: Kebir et al., 2018. Humanized mouse model of Rasmussen’s encephalitis supports the immune-mediated hypothesis. Journal of Clinical Investigation.
Article by Cheleka AM Mpande











