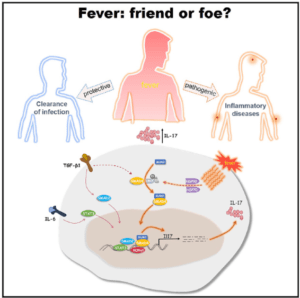
Fever also called as pyrexia, is a pathophysiological response to infections, autoimmune diseases or cancer. It is an evolutionary conserved process that protects from infection through inhibiting pathogen growth and boosting protective immunity. Fever activates the innate immune system, by stimulating the release of neutrophils into the periphery, production of cytokines and nitric oxide from macrophages and/or dendritic cells, promotion of leukocyte trafficking, and enhancement of their phagocytic, bacteriolytic, cytolytic or antigen presentation functions. The regulation of innate immunity in fever suggests its immuno-modulatory function. However, its role in differentiation of T cells associated with inflammatory diseases is elusive.
Researchers led by Prof Chen Dong from Tsinghua University (Beijing, China) have demonstrated that increased body temperature critically regulates the differentiation and pathogenicity of Th17, a subset of T cells which have been associated with inflammation and exacerbation of inflammatory diseases*. Compared to the other T cell subsets i.e Th1, Th2 and Treg cells, febrile temperature (39.50C) selectively enhanced the differentiation of Th17 cells in HSP70 and HSP90 dependent manner. As studied by gene expression analysis (RNA seq), Th17 cells cultured at 39.50C showed increased expression of genes associated with pathogenic phenotype and supported enhanced neutrophil infiltration to the lungs in an acute lung inflammation model. The authors have shown that the transcription factor SMAD4 is indispensable for the febrile-temperature mediated Th17 cell differentiation in vivo and its deficiency reduced the symptoms associated with experimental autoimmune encephalomyelitis. The SMAD4 specifically undergo enhanced SUMOylation at febrile temperature in Th17 cells and facilitate its nuclear translocation. Whole genome sequence analysis of wild type (Smad4fl/fl) and Smad4∆CD4 (Smad4fl/flCd4Cre) Th17 cells showed that Smad4 regulate the genes critical for the differentiation and effector function of Th17 cells, such as Il17a, Il17f, Il21, Il1r1, Il23r, Tbx21, Nfatc1, Nr4a2 etc. Further the genome-wide SMAD4 ChIP-seq assay showed that SMAD4 regulates Th17 differentiation at febrile temperatures, likely through direct binding to the target gene loci.
The present study has demonstrated that Th17 cells generated under increased temperature are more pro-inflammatory and unravelled that SMAD4 is indispensable for Th17 differentiation under the control of environmental cytokines and higher temperatures. In summary, this study has provided the insights into the contribution of fever in the immune-regulation during disease settings where fever is a common physiological response.
Journal article: Wang et al., 2020. Febrile temperature critically controls the differentiation and pathogenicity of T helper 17 Cells. Immunity 52, 1–14.
Article by Rushikesh Patil
Note: * Th17 cells have also been associated with protective immune responses during bacterial and fungal infections.