With the advancement in cancer research, there have several developments made with regards to new therapeutic interventions that can help boost the immune system and its protective response to tumour cells. These interventions fall under the category of immunotherapies. Other interventions that have been developed to address cancer development and cure involve the generation of new drug targets.
A key element that would go a long way in addressing how effective these interventions are in cancer patients and just how useful they are is the need for biomarkers, one that can be associated with clinical prognosis of the cancer and/or severe immune-related adverse effects (irAEs) of the drugs are areas of active investigation.
To address this gap that could eventually help us make current therapeutic interventions helpful for even more cancer patients, the authors made use of a high precision approach called mimotope variation analysis (MVA), a next generation random peptide phage display method to help unpack cancer therapy-associated antibody immune response at epitope resolution. The target of their research was on antibody responses in particular because a lot more focus on the fight against cancer has been focussed on the role of T-cells, however publications have come up to show the role of B-cells and antibody responses in controlling tumour progression.
In this paper, the authors hypothesized that pre-existing and treatment-induced antibodies against specific antigen targets could reflect the response elicited by anti-tumour drugs and that this response could be predictive of cancer immunogenicity and therefore, sensitivity to immune therapy. This hypothesis was tested by analysing the antimelanoma antibody response in the sera of samples coming from patients in a phase II clinical trial for non-small cell lung cancer (NSCLC) receiving autologous DC therapy based on allogenic melanoma cell lysate (MelCancerVac®). They corroborated their findings using melanoma-specific antigen profiles with those from a group of patients with unresectable metastatic melanoma receiving anti-PD-1 (pembrolizumab) treatment as a part of their standard-of-care.
One of the key findings of the paper, related to Figure 1, was that the authors did find out that antibody responses induced by immunotherapy actually differ per individual. Different individuals with the same disease and immunotherapy background has significant differences in the makeup and strength of the dominant antibody response to different peptide antigens. One of the parameters evaluated in this analysis was the Cosine similarity index (CSI), which was a measure of similarity between the samples. CSI was calculated to compare the top antibody response by analysis of seroresponse to 2500 peptides with the highest antibody reactivity in cohort samples.
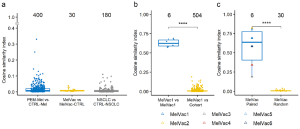
Figure 1: Top antibody response is individual-specific. a–c “The top antibody response was analyzed using cosine similarity indices (CSI) by comparing the composition and abundance values of the 2500 most IgG-bound peptides in each sample to that of the rest of the cohort in pairs (Supplementary Data 1). CSI values (range from 0 to 1, y-axis) between samples belonging to the indicated groups (x-axis) are depicted. Numbers above boxplots indicate the number of comparison pairs shown as dots. Comparisons of samples to themselves (CSI= 1) are not depicted. Comparisons between different individuals are indicated with circles while, comparison of the samples of the same patient are indicated with triangles. a Pairwise comparison between study groups and their matched controls. PEM-Mel – melanoma patients receiving pembrolizumab treatment (n = 5); CTRL-Mel – healthy controls for melanoma group (n = 80); MelVac – NSCLC patients who received MelCancerVac® vaccine (n = 6); MelVac-CTRL – paired samples of MelVac group taken before vaccination (n = 6); NSCLC – non-small cell lung cancer patients (n = 18); CTRL-NSCLC – non-cancer controls for NSCLC group (n = 10). b Pairwise comparisons of the 4 longitudinal samples of one NSCLC patient, who received 35 doses of MelCancerVac® and remained with stable disease (Supplementary Table 2), to the 4 samples themselves (MelVac1 vs MelVac1) and to the rest of the study cohort (n = 126 samples, MelVac1 vs Cohort). c Pairwise comparisons of pre- and post-vaccination immunoprofiles of vaccinated NSCLC patients (n = 6). MelVac Paired – comparison of pre- and postvaccination samples of the same patient; MelVac Random – comparison of the pre-vaccination sample of one patient to the post-vaccination samples of all 5 other patients. Two-sided Wilcoxon Rank Sum test, **** p < 0.0001, p-values not adjusted for multiple comparisons.”
The authors believe that the results point to distinct humoral immune functions connected to the kind of therapy that cancer patients are on. When getting down to specific antibody types, it was determined that IgG responses to specific epitopes of a subset of melanoma-antigens was associated with dendritic cell vaccine treatments in lung cancer. Patients that received MelCancerVac® even showed prior responses to some epitopes which was then just further enhanced by treatments. From this the authors do conclude that these antibody responses could be potential biomarkers associated with anti-melanoma immunity already present at the pre-treatment stage.
The authors had to say in line with their results; “Our data provide support for the use of epitopes of tumour antigens as biomarkers for patient stratification and in immune monitoring. Future studies will elaborate on the connection between antibody profiles and ICI treatment outcomes”. Due to certain limitations in their study and just how specific they were in their approaches, the authors caution that they are had to be careful with their conclusions and said; “we can implicate an epitope by similarity to a self-protein but it is premature to exclude neoepitopes, cryptic epitopes, and metagenome associated epitopes for which we do not have data. Finally, although this study harnessed the use of samples from a phase II clinical trial for the discovery of blood biomarkers, due to the limited number of samples further clinical studies are warranted”.
Journal article: Rähni, A., et al., 2022. Melanoma-specific antigen-associated antitumor antibody reactivity as an immune-related biomarker for targeted immunotherapies. Communications Medicine.
Summary by Vanessa Muwanga










